Difference between revisions of "Ethylenediamine"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
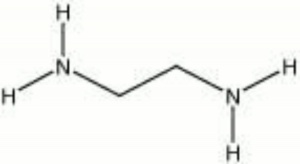
| − | A colorless liquid with a ammoniacal odor. Ethylenediamine is strongly alkaline and will absorb [ | + | A colorless liquid with a ammoniacal odor. Ethylenediamine is strongly alkaline and will absorb [[carbon dioxide]] from the air forming a nonvolatile carbonate. Ethylenediamine is used in developing baths for color photographs. It is also used as a solvent for [[casein]], [[albumin]], [[shellac]], and [[sulfur]]. Other applications include use as a textile lubricant and rubber stabilizer. |
| − | + | [[[SliderGallery rightalign|ethylenediamine.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
1,2-diaminoethane; 1,2-ethanediamine; ethylene diamine | 1,2-diaminoethane; 1,2-ethanediamine; ethylene diamine | ||
| − | + | == Risks == | |
| − | == | + | * Toxic by inhalation and skin absorption. |
| + | * Irritating to skin and eye. | ||
| + | * Flammable. Flash point = 34C. Gives off toxic fumes in when burning. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=E479500&productDescription=ETHYLENDIAMIN+ANHYD+CERT+500ML&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water and ethanol. Slightly soluble in ether. Insoluble in benzene. | Soluble in water and ethanol. Slightly soluble in ether. Insoluble in benzene. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 8.5 | + | | 8.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9 | + | | 0.9 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 116 | + | | 116 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 16:27, 5 August 2022
Description
A colorless liquid with a ammoniacal odor. Ethylenediamine is strongly alkaline and will absorb Carbon dioxide from the air forming a nonvolatile carbonate. Ethylenediamine is used in developing baths for color photographs. It is also used as a solvent for Casein, Albumin, Shellac, and Sulfur. Other applications include use as a textile lubricant and rubber stabilizer.
Synonyms and Related Terms
1,2-diaminoethane; 1,2-ethanediamine; ethylene diamine
Risks
- Toxic by inhalation and skin absorption.
- Irritating to skin and eye.
- Flammable. Flash point = 34C. Gives off toxic fumes in when burning.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water and ethanol. Slightly soluble in ether. Insoluble in benzene.
| Composition | NH2CH2CH2NH2 |
|---|---|
| CAS | 107-15-3 |
| Melting Point | 8.5 C |
| Density | 0.9 g/ml |
| Molecular Weight | mol. wt. = 60.1 |
| Refractive Index | 1.454 |
| Boiling Point | 116 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3841
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.454