Difference between revisions of "Lithium carbonate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
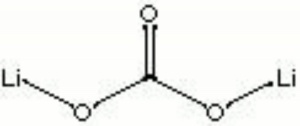
[[[SliderGallery rightalign|lithium carbonate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|lithium carbonate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Corrosive to skin, eyes, and membranes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC413261000&productDescription=LITHIUM+CARBONATE+FOR+A+100GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in dilute acid. Slightly soluble in water. Insoluble in ethanol. | Soluble in dilute acid. Slightly soluble in water. Insoluble in ethanol. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 720 | + | | 720 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.111 | + | | 2.111 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 1200(dec) | + | | 1200 C (dec) |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552 | ||
Revision as of 11:01, 16 September 2022
Description
A white crystalline compound. Lithium carbonate is used as a Flux in the manufacture of Ceramic and Porcelain glazes, and enamels. It is also used to make luminescent paints, varnishes, and dyes.
Synonyms and Related Terms
dilithium carbonate; Camcolit; Candamide; Carbolith; Eskalith; Limas; Lithane; Lithobid; Lithonate; Lithotabs; Plenur; Priadel
Risks
- Corrosive to skin, eyes, and membranes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in dilute acid. Slightly soluble in water. Insoluble in ethanol.
| Composition | Li2CO3 |
|---|---|
| CAS | 554-13-2 |
| Melting Point | 720 C |
| Density | 2.111 g/ml |
| Molecular Weight | mol. wt. = 73.9 |
| Boiling Point | 1200 C (dec) |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979