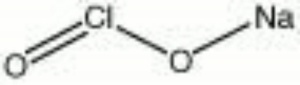
Sodium chlorite
Jump to navigation
Jump to search
Description
White, slightly hygroscopic crystals. Sodium chlorite is a powerful oxidizing agent. A dilute aqueous solution produces chlorous acid which is used as a mild Bleaching agent for textiles. Sodium chlorite is also used for the dilignification of paper pulp.
Risks
- Very strong oxidizer.
- Fire and explosion hazard in contact with organic materials.
- Highly corrosive to tissues.
- Causes corrosion and burns.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water.
| Composition | NaClO2 |
|---|---|
| CAS | 7758-19-2 |
| Melting Point | 180-200 C (dec) |
| Molecular Weight | mol. wt. = 90.44 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 686
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8793