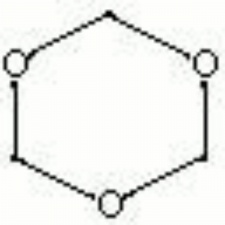
Trioxane
Jump to navigation
Jump to search
Description
White, crystalline solid with a Formaldehyde odor. Trioxane is a trimer of anhydrous formaldehyde in a linked ring structure. It is used as a tanning agent and as a source for formaldehyde. Pellets of trioxane are used as a solid fuel in portable heating units.
Synonyms and Related Terms
sym-trioxane; 1,3,5-trioxane; 1,3,5-trioxacyclohexane; metaformaldehyde; trioxymethylene; triformal
Risks
- Fire risk. Ignites readily.
- Burns with an odorless hot flame.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water and polar organic solvents. Insoluble in aliphatic hydrocarbons. Decomposes with acids to produce formaldehyde.
| Composition | (HCHO)3 |
|---|---|
| CAS | 110-88-3 |
| Melting Point | 62 C |
| Density | 1.17 g/ml |
| Molecular Weight | mol. wt. = 90.08 |
| Boiling Point | 115 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 23
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9863