Difference between revisions of "Dithizone"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 38: | Line 38: | ||
N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'' Archetype Publications, London, 2000, p.96. | N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'' Archetype Publications, London, 2000, p.96. | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3395 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3395 | ||
Revision as of 19:50, 30 April 2016
Description
Blue-black crystalline powder used as a colorimetric reagent for the detction of Zinc in metal objects, corrosion products, and pigments (Odegaard et al 2000). Zinc reacts with dithizone to produce a pink-red residue. Dithizone also reacts with Silver (violet), Copper (dark yellow), Lead (dark red), and Mercury (orange).
Synonyms and Related Terms
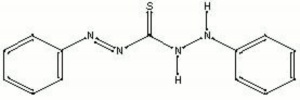
diphenylthiocarbazone; phenyldiazenecarbothioic acid 2-phenylhydrazide
Other Properties
Insoluble in water. Soluble in carbon tetrachloride, chloroform Slightly soluble in alcohols.
| Composition | C13H12N4S |
|---|---|
| CAS | 60-10-6 |
| Melting Point | 168 (dec) |
| Molecular Weight | mol. wt. = 256.32 |
Hazards and Safety
Contact may cause irritation.
Fisher Scientific: MSDS
Additional Information
N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology Archetype Publications, London, 2000, p.96.
Sources Checked for Data in Record
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #3395
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000