Difference between revisions of "Xylose"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
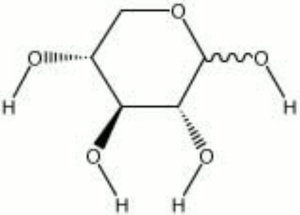
| − | A pentose sugar with a sweet taste that is used as a sugar substitute. Xylose is a dextrorotatory white powder composed of colorless needle or prism crystals. It is derived from the hydrolysis of wood ([ | + | A pentose sugar with a sweet taste that is used as a sugar substitute. Xylose is a dextrorotatory white powder composed of colorless needle or prism crystals. It is derived from the hydrolysis of wood ([[maple|maple]] and [[cherry%20wood|cherry]]), [[straw|straw]], corn cobs, peanut shells, cottonseed hulls and wood pulp waste. Xylose is used in dyeing, tanning leather, and as a diabetic food. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 14:19, 9 May 2016
Description
A pentose sugar with a sweet taste that is used as a sugar substitute. Xylose is a dextrorotatory white powder composed of colorless needle or prism crystals. It is derived from the hydrolysis of wood (Maple and cherry), Straw, corn cobs, peanut shells, cottonseed hulls and wood pulp waste. Xylose is used in dyeing, tanning leather, and as a diabetic food.
Synonyms and Related Terms
wood sugar; Xylomed; Xylopfan
Other Properties
Soluble in water, pyridine, ethanol.
| Composition | C5H10O5 |
|---|---|
| CAS | 58-86-6 |
| Melting Point | 144-145 |
| Density | 1.525 |
| Molecular Weight | mol. wt. = 150.14 |
Hazards and Safety
Combustible.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 781
- G.G. Hawley, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 6th ed., 1961
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Xylose (accessed Mar. 10, 2006)