Difference between revisions of "Cobaltous phosphate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 4: | Line 4: | ||
A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or [[cobalt violet, deep|deep cobalt violet]], was first prepared in 1859. It is a permanent pigment but it has low tinting strength. It dries quickly in [[oil paint|oil paints]]. Cobaltous phosphate is also used as a colorant in [[glass]], [[glaze|glazes]], [[enamel, inorganic|enamels]], and [[plastic|plastics]]. | A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or [[cobalt violet, deep|deep cobalt violet]], was first prepared in 1859. It is a permanent pigment but it has low tinting strength. It dries quickly in [[oil paint|oil paints]]. Cobaltous phosphate is also used as a colorant in [[glass]], [[glaze|glazes]], [[enamel, inorganic|enamels]], and [[plastic|plastics]]. | ||
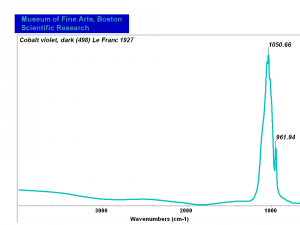
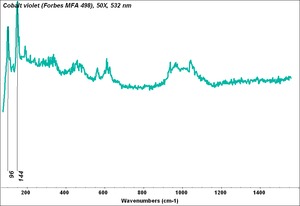
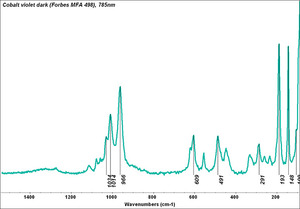
| + | [[[SliderGallery rightalign|Cobalt Violet, dark (498).PNG ~FTIR (MFA) (Forbes 498)|Cobalt violet (Forbes MFA 498), 50X, 532 nm.TIF~Raman (MFA) (532nm)|Cobalt violet dark (Forbes MFA 498), 785nm resize.tif~Raman (MFA) (785nm)|f498sem.jpg~SEM (MFA)|f498edsbw.jpg~EDS (MFA)]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 07:34, 15 October 2019
Description
A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or deep cobalt violet, was first prepared in 1859. It is a permanent pigment but it has low tinting strength. It dries quickly in oil paints. Cobaltous phosphate is also used as a colorant in Glass, glazes, enamels, and plastics.
Synonyms and Related Terms
cobalt phosphate; Pigment Violet 14; CI 77360; fosfato de cobalto (Esp., Port.); violeta de cobalto (Esp.); phosphate de cobalt (Fr.); fosfato di cobalto (It.); cobalt violet, deep
Other Properties
Soluble in mineral acids. Insoluble in water.
| Composition | Co3(PO4)2 - 8H2O |
|---|---|
| CAS | 13455-36-2 |
| Density | 2.769 |
| Molecular Weight | mol. wt. = 366.74 |
Hazards and Safety
Skin contact may cause allergies, especially on elbows, neck and ankles. Chronic inhalation may cause asthma. Ingestion may cause vomiting, diarrhea and the sensation of hotness.
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2508
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980