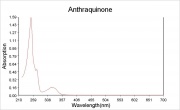
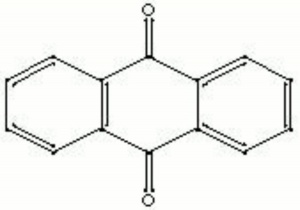
Difference between revisions of "Anthraquinone"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|anthraquinone.jpg~Chemical structure]]] | [[[SliderGallery rightalign|anthraquinone.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | == | + | Combustible. Flash point = 185C (365 F). Causes skin irritation. |
| + | |||
| + | Fisher Scientific: [https://fscimage.fishersci.com/msds/97262.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in ethanol, ether and acetone. Insoluble in water. | Soluble in ethanol, ether and acetone. Insoluble in water. | ||
| Line 35: | Line 39: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 284 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 284 | ||
| Line 53: | Line 51: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 726 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 726 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Anthraquinone (Accessed Mar. 20, 2006) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 13:13, 30 October 2020
Description
Yellow, needle-like crystals that are derived from Anthracene or Phthalic anhydride. Anthraquinone was first sold commercially in 1901. It was used as the starting material in the manufacture of many synthetic dyes, such as alizarin. Anthraquinone may be detected by the appearance of a red color on treatment with alkali, Zinc powder, and Water.
Synonyms and Related Terms
anthroquinone (sp); 9,10-anthracenedione; 9,10-anthraquinone; 9,10-dioxoanthracene; Morkit; Anthradione; Anthrachinon (Deut.); anthraquinon (Fr.); antrachinone (It.)
Risks
Combustible. Flash point = 185C (365 F). Causes skin irritation.
Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, ether and acetone. Insoluble in water.
| Composition | C6H4(CO)2C6H4 |
|---|---|
| CAS | 84-65-1 |
| Melting Point | 286 |
| Density | 1.419-1.438 |
| Molecular Weight | mol. wt.=208.05 |
| Boiling Point | 379-381 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 284
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 726
- Wikipedia: http://en.wikipedia.org/wiki/Anthraquinone (Accessed Mar. 20, 2006)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997