Difference between revisions of "Cobaltous phosphate"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or [ | + | A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or [[cobalt violet, deep|deep cobalt violet]], was first prepared in 1859. It is a permanent pigment but it has low tinting strength. It dries quickly in [[oil paint|oil paints]]. Cobaltous phosphate is also used as a colorant in [[glass]], [[glaze|glazes]], [[enamel, inorganic|enamels]], and [[plastic|plastics]]. |
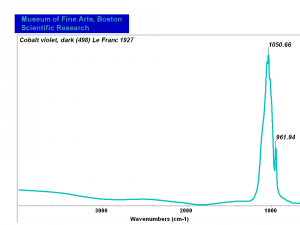
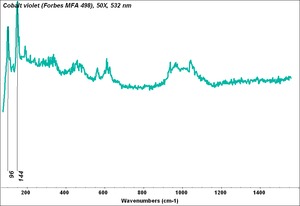
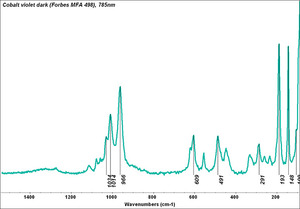
| + | [[[SliderGallery rightalign|Cobalt Violet, dark (498).PNG ~FTIR (MFA) (Forbes 498)|Cobalt violet (Forbes MFA 498), 50X, 532 nm.TIF~Raman (MFA) (532nm)|Cobalt violet dark (Forbes MFA 498), 785nm resize.tif~Raman (MFA) (785nm)|f498sem.jpg~SEM (MFA)|f498edsbw.jpg~EDS (MFA)]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
cobalt phosphate; Pigment Violet 14; CI 77360; fosfato de cobalto (Esp., Port.); violeta de cobalto (Esp.); phosphate de cobalt (Fr.); fosfato di cobalto (It.); cobalt violet, deep | cobalt phosphate; Pigment Violet 14; CI 77360; fosfato de cobalto (Esp., Port.); violeta de cobalto (Esp.); phosphate de cobalt (Fr.); fosfato di cobalto (It.); cobalt violet, deep | ||
| + | == Risks == | ||
| − | == | + | * Skin contact may cause allergies, especially on elbows, neck and ankles. |
| + | * Chronic inhalation may cause asthma. | ||
| + | * Ingestion may cause vomiting, diarrhea and the sensation of hotness. | ||
| + | * NIH: [https://pubchem.ncbi.nlm.nih.gov/compound/Cobalt_II_-phosphate Information shet] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in mineral acids. Insoluble in water. | Soluble in mineral acids. Insoluble in water. | ||
| Line 21: | Line 28: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.769 | + | | 2.769 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 27: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:34, 30 May 2022
Description
A medium to strong violet pigment with a reddish hue. Cobaltous phosphate, or deep cobalt violet, was first prepared in 1859. It is a permanent pigment but it has low tinting strength. It dries quickly in oil paints. Cobaltous phosphate is also used as a colorant in Glass, glazes, enamels, and plastics.
Synonyms and Related Terms
cobalt phosphate; Pigment Violet 14; CI 77360; fosfato de cobalto (Esp., Port.); violeta de cobalto (Esp.); phosphate de cobalt (Fr.); fosfato di cobalto (It.); cobalt violet, deep
Risks
- Skin contact may cause allergies, especially on elbows, neck and ankles.
- Chronic inhalation may cause asthma.
- Ingestion may cause vomiting, diarrhea and the sensation of hotness.
- NIH: Information shet
Physical and Chemical Properties
Soluble in mineral acids. Insoluble in water.
| Composition | Co3(PO4)2 - 8H2O |
|---|---|
| CAS | 13455-36-2 |
| Density | 2.769 g/ml |
| Molecular Weight | mol. wt. = 366.74 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2508
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980