Difference between revisions of "Dipentene"
Jump to navigation
Jump to search
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless liquid with a lemon-like odor that occurs naturally in oils of lemon, orange, caraway, dill, and bergamot. Dipentene, or limonene, is used as a wetting and dispersing agent in liquid [[soap|soaps]], [[ink|inks]], [[essential oil|perfumes]], [[paint|paints]], [[varnish|varnishes]], [[floor finish|floor finishes]], and [[furniture polish|furniture polishes]]. It is also used as a solvent for [[alkyd resin|alkyd resins]], [[rosin]], [[wax|waxes]], and [[rubber | + | A colorless liquid with a lemon-like odor that occurs naturally in oils of lemon, orange, caraway, dill, and bergamot. Dipentene, or limonene, is used as a wetting and dispersing agent in liquid [[soap|soaps]], [[ink|inks]], [[essential oil|perfumes]], [[paint|paints]], [[varnish|varnishes]], [[floor finish|floor finishes]], and [[furniture polish|furniture polishes]]. It is also used as a solvent for [[alkyd resin|alkyd resins]], [[rosin]], [[wax|waxes]], and [[rubber|rubber]] compounds. |
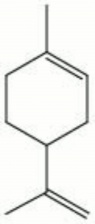
| − | + | [[[SliderGallery rightalign|dipentene.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
cinene; limonene; cajeputene; kautschin; dl-p-mentha-1,8-diene; 1-methyl-4-(1-methylethenyl)cyclohexene | cinene; limonene; cajeputene; kautschin; dl-p-mentha-1,8-diene; 1-methyl-4-(1-methylethenyl)cyclohexene | ||
| − | + | == Risks == | |
| − | == | + | * Skin irritant. |
| + | * Flammable. Flash point = 42C (107.6F) | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/97497.htm MSDS] | ||
| + | ==Physical and Chemical Properties== | ||
Miscible with ethanol. Insoluble in water. | Miscible with ethanol. Insoluble in water. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -97 | + | | -97 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.8402 | + | | 0.8402 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 175.5-176.5 | + | | 175.5-176.5 C |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* R. Mayer, ''The Artist's Handbook of Materials and Techniques'', Viking Press, New York, 1981 | * R. Mayer, ''The Artist's Handbook of Materials and Techniques'', Viking Press, New York, 1981 | ||
Latest revision as of 16:02, 21 July 2022
Description
A colorless liquid with a lemon-like odor that occurs naturally in oils of lemon, orange, caraway, dill, and bergamot. Dipentene, or limonene, is used as a wetting and dispersing agent in liquid soaps, inks, perfumes, paints, varnishes, floor finishes, and furniture polishes. It is also used as a solvent for alkyd resins, Rosin, waxes, and Rubber compounds.
Synonyms and Related Terms
cinene; limonene; cajeputene; kautschin; dl-p-mentha-1,8-diene; 1-methyl-4-(1-methylethenyl)cyclohexene
Risks
- Skin irritant.
- Flammable. Flash point = 42C (107.6F)
- Fisher Scientific: MSDS
Physical and Chemical Properties
Miscible with ethanol. Insoluble in water.
| Composition | C10H16 |
|---|---|
| CAS | 138-86-3 |
| Melting Point | -97 C |
| Density | 0.8402 g/ml |
| Molecular Weight | mol. wt. = 136.24 |
| Boiling Point | 175.5-176.5 C |
Resources and Citations
- R. Mayer, The Artist's Handbook of Materials and Techniques, Viking Press, New York, 1981
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5518
- MSDS Sheet Comment: Fisher Scientific: flash point = 46 C, bp= 165C