Difference between revisions of "Lauric acid"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A fatty acid with a 12 carbon chain. Lauric acid occurs naturally in [ | + | A fatty acid with a 12 carbon chain. Lauric acid occurs naturally in [[coconut oil]] and [[laurel]] oil. At room temperature, lauric acid is a solid. The waxy compound is used to make [[alkyd resin|alkyd resins]], [[wetting agent|wetting agents]], [[soap|soaps]], [[detergent|detergents]], and [[insecticide|insecticides]]. |
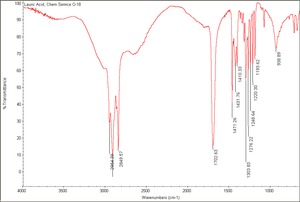
| − | + | [[[SliderGallery rightalign|Lauric Acid, Chem Service O-18.TIF~FTIR (MFA)]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
dodecanoic acid; laurostearic acid; dodecoic acid | dodecanoic acid; laurostearic acid; dodecoic acid | ||
| − | + | == Risks == | |
| − | == | + | * Combustible. Flash point = 112 C. |
| + | * Contact may cause skin and eye irritation | ||
| + | * CDH Fine Chemicals: [https://www.cdhfinechemical.com/images/product/msds/19_1182812426_LAURICACID-CASNO.143-07-7-MSDS.pdf SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in benzene and ether. Slightly soluble in ethanol. Insoluble in water. | Soluble in benzene and ether. Slightly soluble in ethanol. Insoluble in water. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 44 | + | | 44 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.869 | + | | 0.869 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 160-165 | + | | 160-165 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5396 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:37, 16 September 2022
Description
A fatty acid with a 12 carbon chain. Lauric acid occurs naturally in Coconut oil and Laurel oil. At room temperature, lauric acid is a solid. The waxy compound is used to make alkyd resins, wetting agents, soaps, detergents, and insecticides.
Synonyms and Related Terms
dodecanoic acid; laurostearic acid; dodecoic acid
Risks
- Combustible. Flash point = 112 C.
- Contact may cause skin and eye irritation
- CDH Fine Chemicals: SDS
Physical and Chemical Properties
Soluble in benzene and ether. Slightly soluble in ethanol. Insoluble in water.
| Composition | CH3(CH2)10COOH |
|---|---|
| CAS | 143-07-7 |
| Melting Point | 44 C |
| Density | 0.869 g/ml |
| Molecular Weight | mol. wt. = 200.31 |
| Boiling Point | 160-165 C |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5396
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993