Difference between revisions of "Lithol red"
Jump to navigation
Jump to search
| (3 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
[BASF] A trademark for a series of bright red, metallic salts of a synthetic [[azo dye]]. First patented in 1899 by Paul Julius (BASF), Lithol reds are made by combining Tobias acid (2-naphthylamine-1-sulfonic acid) and beta-naphthol. Variations in colors are obtained by forming the salts with [[sodium]], [[barium]], [[calcium]], and [[strontium]] cations. Lithol reds are inexpensive and widely used in [[printing ink|printing inks]], [[crayon|crayons]], [[enamel, organic|enamels]], stationery, and industrial paints. They are also used as a colorants in some [[plastic|plastics]]. Lithol reds are not lightfast in sunlight and have only been used in student grade artists paints. | [BASF] A trademark for a series of bright red, metallic salts of a synthetic [[azo dye]]. First patented in 1899 by Paul Julius (BASF), Lithol reds are made by combining Tobias acid (2-naphthylamine-1-sulfonic acid) and beta-naphthol. Variations in colors are obtained by forming the salts with [[sodium]], [[barium]], [[calcium]], and [[strontium]] cations. Lithol reds are inexpensive and widely used in [[printing ink|printing inks]], [[crayon|crayons]], [[enamel, organic|enamels]], stationery, and industrial paints. They are also used as a colorants in some [[plastic|plastics]]. Lithol reds are not lightfast in sunlight and have only been used in student grade artists paints. | ||
| − | + | * Na salt: yellowish red, PR 49, CI 15630, water soluble | |
| + | * Ba salt: bluish pink, PR 49:1, CAS 1248-18-6 | ||
| + | * Ca salt: bright red, PR 49:2, CAS 1103-38-4 | ||
| + | * Sr salt: purplish red, PR 49:3, CAS 1103-39-5 | ||
| − | + | == Synonyms and Related Terms == | |
| − | + | Lithol toner; Pigment Red 49; CI 15630; 1-sulfo-beta-naphthalene-azo-beta-naphthol; Harrison Red | |
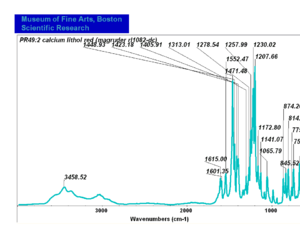
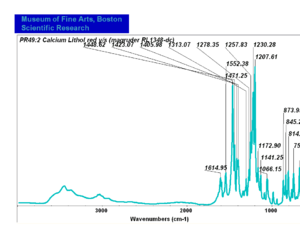
| − | + | [[[SliderGallery rightalign|PR049.1 sun barium lithol 210-4200.TIF~FTIR Ba Sun(MFA)|PR49-1 Barium Lithol red1.PNG~FTIR Ba Magruder(MFA)|PR049.2 sun calcium lithol red 211-4620.TIF~FTIR Ca red(MFA)|PR049.2 sun calcium lithol maroon 211-4980.TIF~FTIR Ca maroon(MFA)|PR49-2 Calcium Lithol red (magruder RL1082-dc).PNG~FTIR Ca Magruder(MFA)|PR49-2 Calcium Lithol red (magruder RL1348-dc).PNG~FTIR Ca y-s Magruder(MFA)]]] | |
| − | == | + | == Risks == |
| − | + | * May cause allergic reactions. | |
| + | * May be contaminated with cancer causing chemicals. | ||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 22: | Line 25: | ||
|- | |- | ||
| PR49:1 || Magruder || barium lithol red|| rl1204-dc || | | PR49:1 || Magruder || barium lithol red|| rl1204-dc || | ||
| + | |- | ||
| + | | PR49:1|| Sun || barium lithol red || 210-2400 || | ||
|- | |- | ||
| PR49:2 || Magruder || calcium lithol red|| rl1082-dc || | | PR49:2 || Magruder || calcium lithol red|| rl1082-dc || | ||
|- | |- | ||
| PR49:2 || Magruder || calcium lithol red y_s|| rl1348-dc || | | PR49:2 || Magruder || calcium lithol red y_s|| rl1348-dc || | ||
| − | |||
| − | |||
|- | |- | ||
| PR49:2 || Sun || calcium lithol red || 211-4620 || | | PR49:2 || Sun || calcium lithol red || 211-4620 || | ||
| Line 35: | Line 38: | ||
|} | |} | ||
| − | == | + | == Physical and Chemical Properties == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | = | + | * Poor lightfastness. |
| + | * Composition = C20H14N2O4S | ||
| − | + | == Resources and Citations == | |
| − | + | * H. A. L. Standeven, "The History and Manufacture of Lithol Red, A Pigment Used by Mark Rothko in his Seagram and Harvard Murals of the 1950s and 1960s" ''Tate Papers'', Issue 10, 2008. [http://www.tate.org.uk/research/tateresearch/tatepapers/08autumn/harriet-a-l-standeven.shtm Link] | |
| − | + | * B.Berrie, S.Q.Lomax, "Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials" in ''Studies in the History of Art'', No.57, National Gallery of Art, Washington DC, 1997. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 62: | Line 54: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | |||
| − | |||
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Latest revision as of 14:41, 15 October 2022
Description
[BASF] A trademark for a series of bright red, metallic salts of a synthetic Azo dye. First patented in 1899 by Paul Julius (BASF), Lithol reds are made by combining Tobias acid (2-naphthylamine-1-sulfonic acid) and beta-naphthol. Variations in colors are obtained by forming the salts with Sodium, Barium, Calcium, and Strontium cations. Lithol reds are inexpensive and widely used in printing inks, crayons, enamels, stationery, and industrial paints. They are also used as a colorants in some plastics. Lithol reds are not lightfast in sunlight and have only been used in student grade artists paints.
- Na salt: yellowish red, PR 49, CI 15630, water soluble
- Ba salt: bluish pink, PR 49:1, CAS 1248-18-6
- Ca salt: bright red, PR 49:2, CAS 1103-38-4
- Sr salt: purplish red, PR 49:3, CAS 1103-39-5
Synonyms and Related Terms
Lithol toner; Pigment Red 49; CI 15630; 1-sulfo-beta-naphthalene-azo-beta-naphthol; Harrison Red
Risks
- May cause allergic reactions.
- May be contaminated with cancer causing chemicals.
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
| PR49:1 | Magruder | barium lithol red | rl1204-dc | |
| PR49:1 | Sun | barium lithol red | 210-2400 | |
| PR49:2 | Magruder | calcium lithol red | rl1082-dc | |
| PR49:2 | Magruder | calcium lithol red y_s | rl1348-dc | |
| PR49:2 | Sun | calcium lithol red | 211-4620 | |
| PR49:2 | Sun | calcium lithol maroon | 211-4980 |
Physical and Chemical Properties
- Poor lightfastness.
- Composition = C20H14N2O4S
Resources and Citations
- H. A. L. Standeven, "The History and Manufacture of Lithol Red, A Pigment Used by Mark Rothko in his Seagram and Harvard Murals of the 1950s and 1960s" Tate Papers, Issue 10, 2008. Link
- B.Berrie, S.Q.Lomax, "Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials" in Studies in the History of Art, No.57, National Gallery of Art, Washington DC, 1997.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979