Difference between revisions of "Gypsum"
| (11 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:1995.739-E12832CR-d1.jpg|thumb|]] | + | [[File:1995.739-E12832CR-d1.jpg|thumb|Stone vessel<br>MFA# 1995.739]] |
== Description == | == Description == | ||
| − | + | [[File:35.731-SC80319.jpg|thumb|Gypsum relief<br>MFA# 35.731]] | |
| − | A soft, transparent, easily cleaved mineral composed of [[calcium sulfate, dihydrate|hydrated calcium sulfate]]. Gypsum is found as twinned monoclinic crystals, called [[selenite]], or silky fibrous crystals, called [[satin spar]]. Massive blocks of fine-grain white, translucent gypsum are called [[alabaster]] and have been used since ancient times for carved ornamental objects and statuary. Gypsum is a commonly found mineral associated with sedimentary rock and deposits from seas, lakes, and volcanic springs ([[gypcrete]]). For a long time, gypsum quarries in the Montmartre district of Paris supplied the starting material for the burnt gypsum that was, and still is, called [[plaster of Paris]]. Raw gypsum is used for carvings ([[alabaster]]), for wallboards ([[ | + | A soft, transparent, easily cleaved mineral composed of [[calcium sulfate, dihydrate|hydrated calcium sulfate]]. Gypsum is found as twinned monoclinic crystals, called [[selenite]], or silky fibrous crystals, called [[satin spar]]. Massive blocks of fine-grain white, translucent gypsum are called [[alabaster]] and have been used since ancient times for carved ornamental objects and statuary. Gypsum is a commonly found mineral associated with sedimentary rock and deposits from seas, lakes, and volcanic springs ([[gypcrete]]). For a long time, gypsum quarries in the Montmartre district of Paris supplied the starting material for the burnt gypsum that was, and still is, called [[plaster of Paris]]. Raw gypsum is used for carvings ([[alabaster]]), for wallboards ([[Sheetrock]]), as a filler in paper ([[crown filler]]), as a paint pigment ([[terra alba]]) and as an ingredient in [[portland cement]]. Finely ground gypsum was mixed with rabbit skin glue and used as [[gesso]]. |
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.) | native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.) | ||
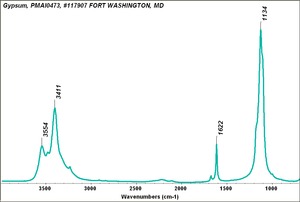
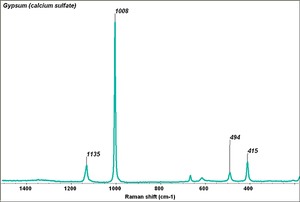
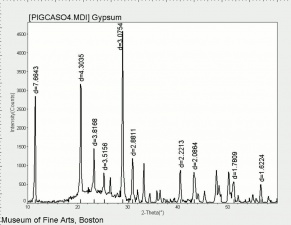
| + | [[[SliderGallery rightalign|Gypsum PMA.TIF~FTIR (PMA)|Gypsum (calcium sulfate) copy.tif~Raman (MFA)|PIGCASO4.jpg~XRD (MFA)]]] | ||
| − | + | == Risks == | |
| − | == | + | * Inhalation and contact may cause slight allergies. |
| + | * American Gypsum: [https://www.americangypsum.com/sites/default/files/documents/SDS_1.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water. Slightly soluble in glycerol and weak acids. Precipitates as needle-like crystals. Insoluble in most organic solvents. Gypsum fluoresces purple. | Soluble in water. Slightly soluble in glycerol and weak acids. Precipitates as needle-like crystals. Insoluble in most organic solvents. Gypsum fluoresces purple. | ||
| − | + | Microscopic identification = in plane polarized light (PPL), colorless with moderate relief. Perfect cleavage in one direction and good cleavage in two directions. Some samples may contain well-formed rhombic or columnar particles. Some particles exhibit twinning with 'swallowtail' shapes. RI < 1.662. In cross-polarized light (XPL), low birefringence with first-order grays observed. Extinction is inclined. | |
Luster = vitreous, silky or pearly. Streak = white. Fracture = conchoidal to splintery. Euhedral shaped crystals contain numerous inclusions. | Luster = vitreous, silky or pearly. Streak = white. Fracture = conchoidal to splintery. Euhedral shaped crystals contain numerous inclusions. | ||
| Line 32: | Line 33: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 100-150 | + | | 100-150 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.32-2.36 | + | | 2.32-2.36 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 43: | Line 44: | ||
| 1.520; 1.523; 1.530 | | 1.520; 1.523; 1.530 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
[[media:download_file_531.pdf|Characteristics of Common White Pigments]] | [[media:download_file_531.pdf|Characteristics of Common White Pigments]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 70: | Line 59: | ||
File:PLM5 twinning in gypsum particle XPL 200x.jpg|Gypsum particles mixed with wheat starch grains stained with organic red dye, PPL 200x | File:PLM5 twinning in gypsum particle XPL 200x.jpg|Gypsum particles mixed with wheat starch grains stained with organic red dye, PPL 200x | ||
File:PLM5 twinning in gypsum particle PPL 200x.jpg|Gypsum particles mixed with wheat starch grains stained with organic red dye, XPL 200x | File:PLM5 twinning in gypsum particle PPL 200x.jpg|Gypsum particles mixed with wheat starch grains stained with organic red dye, XPL 200x | ||
| − | File:1947-177 residue 3 gypsum particle PPL 400x.jpg | + | File:1947-177 residue 3 gypsum particle PPL 400x.jpg|well-formed gypsum particle with 'swallowtail' morphology, PPL, 400x |
| − | File:1947-177 residue 3 gypsum particle XPL 400x.jpg | + | File:1947-177 residue 3 gypsum particle XPL 400x.jpg|well-formed gypsum particle with 'swallowtail' morphology, PPL, 400x |
| − | |||
</gallery> | </gallery> | ||
| − | == | + | ==Resources and Citations== |
| − | + | * https://www.americangypsum.com/sites/default/files/documents/SDS_1.pdf | |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.519-1.521; beta=1.522-1.523; gamma=1.529-1.530 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.519-1.521; beta=1.522-1.523; gamma=1.529-1.530 | ||
| − | |||
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: "gypsum" [Accessed December 4, 2001 (B/W photo) | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "gypsum" [Accessed December 4, 2001 (B/W photo) | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Gypsum Gypsum] (Accessed Nov. 2, 2005 and Jan 2023) | |
| − | * Wikipedia | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.36 and ref.index =.1.520 ;1.530; 1.523 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 2.36 and ref.index =.1.520 ;1.530; 1.523 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 385 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 385 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | * Sue Fuller, ''Rocks and Minerals'', DK Publishing, Inc., New York City, 1995 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753 | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.31-2.33 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.31-2.33 | ||
Latest revision as of 13:18, 24 January 2023
Description
A soft, transparent, easily cleaved mineral composed of hydrated calcium sulfate. Gypsum is found as twinned monoclinic crystals, called Selenite, or silky fibrous crystals, called Satin spar. Massive blocks of fine-grain white, translucent gypsum are called Alabaster and have been used since ancient times for carved ornamental objects and statuary. Gypsum is a commonly found mineral associated with sedimentary rock and deposits from seas, lakes, and volcanic springs (Gypcrete). For a long time, gypsum quarries in the Montmartre district of Paris supplied the starting material for the burnt gypsum that was, and still is, called Plaster of Paris. Raw gypsum is used for carvings (Alabaster), for wallboards (Sheetrock), as a filler in paper (Crown filler), as a paint pigment (Terra alba) and as an ingredient in Portland cement. Finely ground gypsum was mixed with rabbit skin glue and used as Gesso.
Synonyms and Related Terms
native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.)
Risks
- Inhalation and contact may cause slight allergies.
- American Gypsum: SDS
Physical and Chemical Properties
Soluble in water. Slightly soluble in glycerol and weak acids. Precipitates as needle-like crystals. Insoluble in most organic solvents. Gypsum fluoresces purple.
Microscopic identification = in plane polarized light (PPL), colorless with moderate relief. Perfect cleavage in one direction and good cleavage in two directions. Some samples may contain well-formed rhombic or columnar particles. Some particles exhibit twinning with 'swallowtail' shapes. RI < 1.662. In cross-polarized light (XPL), low birefringence with first-order grays observed. Extinction is inclined.
Luster = vitreous, silky or pearly. Streak = white. Fracture = conchoidal to splintery. Euhedral shaped crystals contain numerous inclusions.
| Composition | CaSO4-2H2O |
|---|---|
| CAS | 1010-14-4 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 100-150 C |
| Density | 2.32-2.36 g/ml |
| Molecular Weight | mol. wt. = 172.2 |
| Refractive Index | 1.520; 1.523; 1.530 |
Comparisons
Characteristics of Common White Pigments
Additional Images
Resources and Citations
- https://www.americangypsum.com/sites/default/files/documents/SDS_1.pdf
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.519-1.521; beta=1.522-1.523; gamma=1.529-1.530
- Encyclopedia Britannica, http://www.britannica.com Comment: "gypsum" [Accessed December 4, 2001 (B/W photo)
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: Gypsum (Accessed Nov. 2, 2005 and Jan 2023)
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.36 and ref.index =.1.520 ;1.530; 1.523
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 385
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Sue Fuller, Rocks and Minerals, DK Publishing, Inc., New York City, 1995
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.31-2.33