Difference between revisions of "Azurite"
(username removed) |
|||
| (21 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:64.632a-SC16047.jpg|thumb| | + | [[File:64.632a-SC16047.jpg|thumb|Azurite jewelry<br>MFA#: 64.632a]] |
== Description == | == Description == | ||
| − | A deep blue mineral composed of [ | + | A deep blue mineral composed of [[basic copper carbonate]] that is naturally found adjacent to the green copper carbonate mineral called [[malachite]]. Azurite and malachite have been used as gemstones and paint pigments since ancient times. They are prepared as pigments by careful selection, grinding, washing, and levigation. Coarsely ground azurite gives a deep blue color while finely ground particles give a lighter more transparent tone. Azurite is lightfast but is sensitive to acids and sulfur fumes. Basic copper carbonate can also be made artificially by coloring [[chalk]] with [[copper sulfate]]. The synthetic pigment, called [[blue verditer]], [[blue bice]], [[Bremen blue]], or ashes blue, tends to have regularly sized particles with rounded edges. Their color is similar to finely ground azurite. |
| − | + | [[File:Azurite.statue.ovr.jpg|thumb|Azurite on bronze]] | |
[[File:azuritedw.jpg|thumb|Azurite]] | [[File:azuritedw.jpg|thumb|Azurite]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
basic copper carbonate (natural); Pigment Blue 30; CI 77420; bleu d'Allemagne (Fr.); Bergblau (Deut.); Bergasur; Azurit (Deut.); azurite (Fr., Port.); azzurrite (It.); azzurro della magna (It.); azurita (Esp.); azurium citramarinum; iwagunjo (Jap.); byaku gunjo (Jap.); shi ging (Chin.); mthing (Tibetan); azurro della magna; azoyritis (Gr.); azuriet (Ned.); blue verditer; mountain blue; ashes blue; blue ash; sky blue; German ash; Bremen blue; blue bice; Armenian stone; lapis armenius; chessylite; blue malachite; mineral blue; basic cupric carbonate; copper blue; chessy copper | basic copper carbonate (natural); Pigment Blue 30; CI 77420; bleu d'Allemagne (Fr.); Bergblau (Deut.); Bergasur; Azurit (Deut.); azurite (Fr., Port.); azzurrite (It.); azzurro della magna (It.); azurita (Esp.); azurium citramarinum; iwagunjo (Jap.); byaku gunjo (Jap.); shi ging (Chin.); mthing (Tibetan); azurro della magna; azoyritis (Gr.); azuriet (Ned.); blue verditer; mountain blue; ashes blue; blue ash; sky blue; German ash; Bremen blue; blue bice; Armenian stone; lapis armenius; chessylite; blue malachite; mineral blue; basic cupric carbonate; copper blue; chessy copper | ||
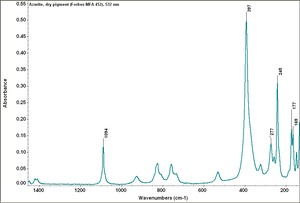
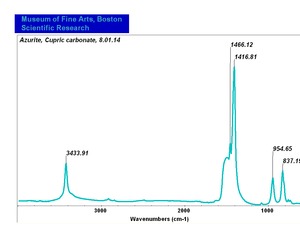
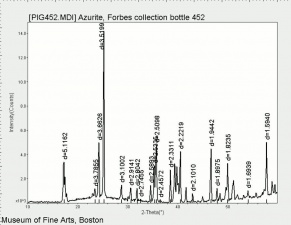
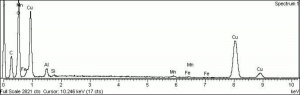
| + | [[[SliderGallery rightalign|Azurite (Forbes MFA 452), 532 nm.TIF~Raman (MFA)|Azurite, cupric carbonate, 8.01.14.TIF~FTIR (MFA)|PIG452.jpg~XRD (MFA)|f452sem.jpg~SEM (MFA)|f452edsbw.jpg~EDS (MFA)]]] | ||
| − | + | == Risks == | |
| − | + | * Skin contact and inhalation may cause irritation or allergic reactions. | |
| − | == | + | * Chronic exposure may cause anemia. |
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Fracture = conchoidal | + | * Insoluble in [[water]], [[ethanol]]. |
| + | * Decomposes in acids with the evolution of [[carbon dioxide]] bubbles. | ||
| + | * Turns black with warm alkalis, [[hydrogen sulfide]], or sulfur fumes. | ||
| + | * Crystal system = monoclinic with massive, prismatic, stalactitic, or tabular crystals | ||
| + | * Cleavage = perfect in one direction; fair to poor in two directions | ||
| + | * Fracture = conchoidal | ||
| + | * Luster = vitreous | ||
| + | * Streak = blue | ||
| + | * Fluorescence = inert | ||
| + | * Pleochroic = moderate to strong in single crystals; pale blue to deep blue | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 33: | Line 38: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.77-3.80 | + | | 3.77-3.80 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.730; 1.838; 1.758 | | 1.730; 1.838; 1.758 | ||
| + | |- | ||
| + | ! scope="row"| Birefringence | ||
| + | | 0.106 - 0.108 (strong) | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | |||
| − | |||
| − | |||
| − | |||
[[media:download_file_499.pdf|Characteristics of Common Blue Pigments]] | [[media:download_file_499.pdf|Characteristics of Common Blue Pigments]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
<gallery> | <gallery> | ||
| − | |||
File:Azurite.corr.det1.jpg|Azurite and malachite on bronze | File:Azurite.corr.det1.jpg|Azurite and malachite on bronze | ||
File:452 azurite.jpg|Azurite | File:452 azurite.jpg|Azurite | ||
| Line 69: | Line 62: | ||
</gallery> | </gallery> | ||
| − | + | ==Resources and Citations== | |
| − | == | + | * Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' ''Minerals'' Vol 8, p. 201 (2018). [https://www.academia.edu/36588315/Mineral_Pigments_in_Archaeology_Their_Analysis_and_the_Range_of_Available_Materials?email_work_card=view-paper Link] |
| − | + | * R. Gettens, and E. West Fitzhugh, "Azurite and Blue Verditer", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Azurite. Retrieved May 24, 2003 | + | * Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/azurite.html Azurite] |
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
| − | * | + | * Mineralogy Database: [http://webmineral.com/data/Azurite.shtml Azurite] |
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Azurite. Retrieved May 24, 2003. | |
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: p. 95 |
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 232 | |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | |
| − | * | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: Pigments | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: Pigments | ||
| − | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | |
| − | * | + | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 |
| − | + | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | |
| − | * | + | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 |
| − | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | |
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 2697 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 2697 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Azurite azurite] (Accessed (Sept 2 2005 and Dec 2022) | |
| − | * Wikipedia | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | |||
| − | * Art and Architecture Thesaurus Online, | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:19, 28 February 2024
Description
A deep blue mineral composed of Basic copper carbonate that is naturally found adjacent to the green copper carbonate mineral called Malachite. Azurite and malachite have been used as gemstones and paint pigments since ancient times. They are prepared as pigments by careful selection, grinding, washing, and levigation. Coarsely ground azurite gives a deep blue color while finely ground particles give a lighter more transparent tone. Azurite is lightfast but is sensitive to acids and sulfur fumes. Basic copper carbonate can also be made artificially by coloring Chalk with Copper sulfate. The synthetic pigment, called Blue verditer, Blue bice, Bremen blue, or ashes blue, tends to have regularly sized particles with rounded edges. Their color is similar to finely ground azurite.
Synonyms and Related Terms
basic copper carbonate (natural); Pigment Blue 30; CI 77420; bleu d'Allemagne (Fr.); Bergblau (Deut.); Bergasur; Azurit (Deut.); azurite (Fr., Port.); azzurrite (It.); azzurro della magna (It.); azurita (Esp.); azurium citramarinum; iwagunjo (Jap.); byaku gunjo (Jap.); shi ging (Chin.); mthing (Tibetan); azurro della magna; azoyritis (Gr.); azuriet (Ned.); blue verditer; mountain blue; ashes blue; blue ash; sky blue; German ash; Bremen blue; blue bice; Armenian stone; lapis armenius; chessylite; blue malachite; mineral blue; basic cupric carbonate; copper blue; chessy copper
Risks
- Skin contact and inhalation may cause irritation or allergic reactions.
- Chronic exposure may cause anemia.
Physical and Chemical Properties
- Insoluble in Water, Ethanol.
- Decomposes in acids with the evolution of Carbon dioxide bubbles.
- Turns black with warm alkalis, Hydrogen sulfide, or sulfur fumes.
- Crystal system = monoclinic with massive, prismatic, stalactitic, or tabular crystals
- Cleavage = perfect in one direction; fair to poor in two directions
- Fracture = conchoidal
- Luster = vitreous
- Streak = blue
- Fluorescence = inert
- Pleochroic = moderate to strong in single crystals; pale blue to deep blue
| Composition | 2CuCO3-Cu(OH)2 |
|---|---|
| CAS | 12069-69-1 |
| Mohs Hardness | 3.5 - 4.0 |
| Density | 3.77-3.80 g/ml |
| Refractive Index | 1.730; 1.838; 1.758 |
| Birefringence | 0.106 - 0.108 (strong) |
Comparisons
Characteristics of Common Blue Pigments
Additional Images
Resources and Citations
- Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' Minerals Vol 8, p. 201 (2018). Link
- R. Gettens, and E. West Fitzhugh, "Azurite and Blue Verditer", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
- Pigments Through the Ages: Azurite
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Mineralogy Database: Azurite
- Encyclopedia Britannica, http://www.britannica.com Comment: Azurite. Retrieved May 24, 2003.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: p. 95
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 232
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: Pigments
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 2697
- Wikipedia: azurite (Accessed (Sept 2 2005 and Dec 2022)
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000