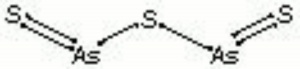Difference between revisions of "Orpiment"
| Line 3: | Line 3: | ||
== Description == | == Description == | ||
[[File:333334 orpiment_2up.jpg|thumb|Orpiment, powdered]] | [[File:333334 orpiment_2up.jpg|thumb|Orpiment, powdered]] | ||
| − | A soft, yellow mineral composed of [[arsenic%20trisulfide|arsenic trisulfide]]. Orpiment, as well as its other two phases | + | A soft, yellow mineral composed of [[arsenic%20trisulfide|arsenic trisulfide]]. Orpiment (As<sub>2</sub>S<sub>3</sub>), as well as its other two phases [[Realgar]] (α-As<sub>2</sub>S<sub>3</sub>) and [[pararealgar]] (As<sub>4</sub>S<sub>4</sub>) have been used widely as pigments. They occur naturally in volcanic fumaroles, hydrothermal veins, and hot springs. Deposits are found in the Czech Republic, Romania (Copalnic), Germany (Andreas-Berg ), Switzerland (Valais), Turkey (Çölemerik), Macedonia, Japan and the United States (Utah, Nevada, Wyoming). Orpiment ranges in color from a bright lemon yellow to orange. It changes to the red crystalline form at 170C. Orpiment was used in many early civilizations at various times, such as Egypt, Syria, Persia, India, and China. It was used as a pigment in European painting from quite early times, including in the Roman period as well as in manuscript illumination and polychrome sculpture to the point that it became almost a standard material on the palette in Venice in the 16th-century. Elsewhere it occurs in Dutch 17th-century painting, particularly flowerpieces, and British and French 18th-century paintings. Its use continued almost up until the present day, though it is no longer commonly used due to its toxicity. Orpiment has good tinting strength but is not considered permanent as it reacts with copper pigments as well as some lead pigments to produce dark copper or lead sulfides. It is a poor drier for oil paints and has a sulfurous odor. It can be rather light sensitive, losing its color on prolonged exposure to light, particularly in aqueous media. Arsenic trisulfide was made synthetically in the 18th-century and sold as king's yellow. The synthetic variety was purer and less expensive. |
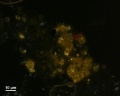
[[File:orpiment C100x.jpg|thumb|orpiment]] | [[File:orpiment C100x.jpg|thumb|orpiment]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 11:55, 29 February 2024
Description
A soft, yellow mineral composed of Arsenic trisulfide. Orpiment (As2S3), as well as its other two phases Realgar (α-As2S3) and Pararealgar (As4S4) have been used widely as pigments. They occur naturally in volcanic fumaroles, hydrothermal veins, and hot springs. Deposits are found in the Czech Republic, Romania (Copalnic), Germany (Andreas-Berg ), Switzerland (Valais), Turkey (Çölemerik), Macedonia, Japan and the United States (Utah, Nevada, Wyoming). Orpiment ranges in color from a bright lemon yellow to orange. It changes to the red crystalline form at 170C. Orpiment was used in many early civilizations at various times, such as Egypt, Syria, Persia, India, and China. It was used as a pigment in European painting from quite early times, including in the Roman period as well as in manuscript illumination and polychrome sculpture to the point that it became almost a standard material on the palette in Venice in the 16th-century. Elsewhere it occurs in Dutch 17th-century painting, particularly flowerpieces, and British and French 18th-century paintings. Its use continued almost up until the present day, though it is no longer commonly used due to its toxicity. Orpiment has good tinting strength but is not considered permanent as it reacts with copper pigments as well as some lead pigments to produce dark copper or lead sulfides. It is a poor drier for oil paints and has a sulfurous odor. It can be rather light sensitive, losing its color on prolonged exposure to light, particularly in aqueous media. Arsenic trisulfide was made synthetically in the 18th-century and sold as king's yellow. The synthetic variety was purer and less expensive.
Synonyms and Related Terms
arsenic trisulfide; Pigment Yellow 39; CI 77085, 77086; orpiment (Eng., Fr., Gr., Ned.); Auripigment (Deut.); Rauschgelb (Deut.); Konigsgelb (Deut.); jaune royal (Fr.); oropimente (Esp.); orpimento (It.); ouropigmento (Port.); arsenous sulfide; king's yellow; arsenic yellow; auripigmentum; Chinese yellow; sunflower yellow
Risks
- Turns black in contact with copper and lead containing pigments.
- Toxic by inhalation and ingestion.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Soluble in acids and alkalis.
- Decomposes slowly in water
- Unstable when mixed with alkaline pigments such as in buon fresco or in combinations with lime white
- Oxidizes to form translucent or white oxides of arsenic.
- Both orpiment and realgar lose color on exposure to light
- Luster = pearly to resinous. Streak = lemon-yellow.
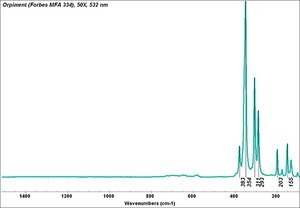
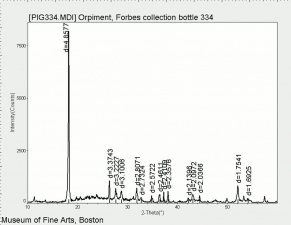
In PPL, orpiment has a strong yellow color and exhibits very high relief. Crystals are coarse-grained (up to 70 microns reported) with perfect cleavage (Eastaugh describes as 'bladed crystals', and 'splinters'), and often a distinct 'cross-hatching' pattern is visible on larger particles. Some particles are fibrous, acicular and/or elongated, and earthy aggregates are also reported. In XPL, particles exhibit high birefringence with pink and green interference colors. Extinction is straight and acicular particles are length-fast. Synthetic orpiment can be differentiated from the naturally occurring mineral form due to its smaller particle size (reported as fine to medium). The synthetic form is also reported to be contaminated with arsenic (III) oxide.
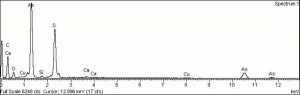
| Composition | As2S3 |
|---|---|
| CAS | 1303-33-9 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 300 C |
| Density | 3.43 g/ml |
| Molecular Weight | mol. wt. = 246.04 |
| Refractive Index | 2.40; 3.02; 2.81 |
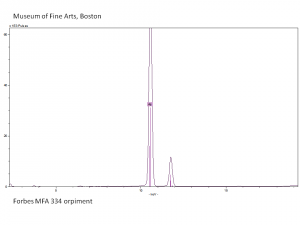
Additional Images
Resources and Citations
- Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' Minerals Vol 8, p. 201 (2018). Link
- E.West FitzHugh, "Orpiment and Realgar", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- Mineralogy Database: Orpiment
- Carolin Rötter, ‘Auripigment’, Restauro, 6 2003, 408-413.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Ashok Roy, Submitted information, November 2007
- Helen Howard, Submitted information, November 2007
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: E.West FitzHugh, "Orpiment and Realgar"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: Jap. name kio and sekio and stone yellow
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 69
- Encyclopedia Britannica, http://www.britannica.com Comment: "orpiment." Accessed 7 Apr. 2005 .
- Pigments Through the Ages - http://webexhibits.org/pigments/indiv/technical/orpiment.html
- McCrone Atlas of Microscopic Particles, Orpiment - http://www.mccroneatlas.com
- Wikipedia: Orpiment (accessed Aug.30 2005 and Aug. 2023)
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
Record content reviewed by EU-Artech, November 2007.