Difference between revisions of "Xylenes"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
(→Risks) |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
A clear, oily, colorless mixture of three liquid isomers: ortho-, meta- and para-xylene. Xylenes are obtained from coal-tar distillation with the meta- isomer being predominate. A large quantity of xylenes are used annually as raw or intermediate materials for synthetic fibers and plastics such as [[polyester%20resin|polyester]]. Xylenes are more toxic than toluene but safer than benzene. Xylene is used as a solvent for [[alkyd%20resin|alkyd resins]], [[lacquer%2C%20synthetic|synthetic lacquers]], [[enamel%2C%20organic|organic enamels]], and [[rubber%20cement|rubber cements]]. It is also used in dye manufacture, as an aviation fuel, and in [[Canada%20balsam|Canada balsam]] for oil-immersion microscopy. | A clear, oily, colorless mixture of three liquid isomers: ortho-, meta- and para-xylene. Xylenes are obtained from coal-tar distillation with the meta- isomer being predominate. A large quantity of xylenes are used annually as raw or intermediate materials for synthetic fibers and plastics such as [[polyester%20resin|polyester]]. Xylenes are more toxic than toluene but safer than benzene. Xylene is used as a solvent for [[alkyd%20resin|alkyd resins]], [[lacquer%2C%20synthetic|synthetic lacquers]], [[enamel%2C%20organic|organic enamels]], and [[rubber%20cement|rubber cements]]. It is also used in dye manufacture, as an aviation fuel, and in [[Canada%20balsam|Canada balsam]] for oil-immersion microscopy. | ||
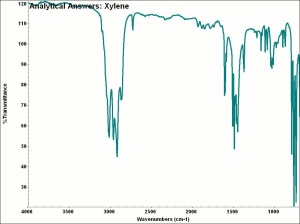
| − | + | [[[SliderGallery rightalign|aaiXYLENE.jpg~FTIR|xylenes.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
xylene; xylol; dimethylbenzene; aromatic naphtha; ortho-xylene; para-xylene | xylene; xylol; dimethylbenzene; aromatic naphtha; ortho-xylene; para-xylene | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Flammable, flash point 27C (81F). | ||
| + | * May be absorbed through the skin, can cause defatting and irritation. Inhalation of large amounts may be fatal (glue sniffer's syndrome). | ||
| + | * CISCO hem: [http://www.ciscochem.com/assets/xylene-sds.pdf SDS] | ||
| + | * EPA lists xylene as hazardous waste; concentrations over 10% must be disposed of appropriately | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in ether and ethanol. Insoluble in water. | Soluble in ether and ethanol. Insoluble in water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | (-25) - 13.2 | + | | (-25) - 13.2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.86 - 0.88 | + | | 0.86 - 0.88 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 39: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 137 - 140 | + | | 137 - 140 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 47: | Line 46: | ||
[[media:download_file_139.pdf|Properties of Common Solvents]] | [[media:download_file_139.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 61: | Line 58: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * Website | + | * Website: www.hants.org.uk/museums/ofr/cmeth_t.html |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.493 (p-xylene); 1.494 (m-xylene) | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.493 (p-xylene); 1.494 (m-xylene) | ||
Latest revision as of 12:21, 17 April 2024
Description
A clear, oily, colorless mixture of three liquid isomers: ortho-, meta- and para-xylene. Xylenes are obtained from coal-tar distillation with the meta- isomer being predominate. A large quantity of xylenes are used annually as raw or intermediate materials for synthetic fibers and plastics such as polyester. Xylenes are more toxic than toluene but safer than benzene. Xylene is used as a solvent for alkyd resins, synthetic lacquers, organic enamels, and rubber cements. It is also used in dye manufacture, as an aviation fuel, and in Canada balsam for oil-immersion microscopy.
Synonyms and Related Terms
xylene; xylol; dimethylbenzene; aromatic naphtha; ortho-xylene; para-xylene
Risks
- Flammable, flash point 27C (81F).
- May be absorbed through the skin, can cause defatting and irritation. Inhalation of large amounts may be fatal (glue sniffer's syndrome).
- CISCO hem: SDS
- EPA lists xylene as hazardous waste; concentrations over 10% must be disposed of appropriately
Physical and Chemical Properties
Soluble in ether and ethanol. Insoluble in water.
| Composition | C6H4(CH3)2 |
|---|---|
| CAS | 1330-20-7 |
| Melting Point | (-25) - 13.2 C |
| Density | 0.86 - 0.88 g/ml |
| Molecular Weight | mol. wt.=106.16 |
| Refractive Index | 1.493-1.494 |
| Boiling Point | 137 - 140 C |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 801
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Website: www.hants.org.uk/museums/ofr/cmeth_t.html
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.493 (p-xylene); 1.494 (m-xylene)