Difference between revisions of "Aluminon"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[[SliderGallery rightalign|aluminon.jpg~Chemical structure]]] | ||
== Description == | == Description == | ||
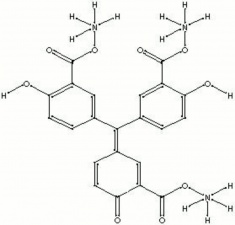
| − | A tradename for shiny, yellow-brown powder that is the ammonium salt of aurin tricarboxylic acid. Aluminon forms brightly colored lakes with [ | + | A tradename for shiny, yellow-brown powder that is the ammonium salt of aurin tricarboxylic acid. Aluminon forms brightly colored lakes with [[aluminum|aluminum]], [[chromium|chromium]], [[iron|iron]], and [[beryllium|beryllium]] salts. It is generally used for the detection of [[alum|alum]] in aqueous extractions of paper. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 8: | ||
ammonium salt of aurine tricarboxylic acid; Lysofon; ammonium aurintricarboxylate; | ammonium salt of aurine tricarboxylic acid; Lysofon; ammonium aurintricarboxylate; | ||
| − | + | == Risks == | |
| − | + | * Skin contact causes irritation. | |
| + | * Harmful if swallowed or inhaled. | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25140A.pdf SDS] | ||
| − | + | == Physical and Chemical Properties == | |
| − | + | * Soluble in water. | |
| + | * The reagent solution is prepared by dissolving 1 g of salt in 1 liter of distilled water. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 220.5 (dec) | + | | 220.5 C (dec) |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | + | * For alum detection procedures see: N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'' Archetype Publications, London, 2000, p. 34. | |
| − | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330 | ||
* ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | * ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | ||
| − | |||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water | ||
Latest revision as of 10:06, 26 April 2022
Description
A tradename for shiny, yellow-brown powder that is the ammonium salt of aurin tricarboxylic acid. Aluminon forms brightly colored lakes with Aluminum, Chromium, Iron, and Beryllium salts. It is generally used for the detection of Alum in aqueous extractions of paper.
Synonyms and Related Terms
ammonium salt of aurine tricarboxylic acid; Lysofon; ammonium aurintricarboxylate;
Risks
- Skin contact causes irritation.
- Harmful if swallowed or inhaled.
- Fisher Scientific: SDS
Physical and Chemical Properties
- Soluble in water.
- The reagent solution is prepared by dissolving 1 g of salt in 1 liter of distilled water.
| Composition | C22H23N3O9 |
|---|---|
| CAS | 569-58-4 |
| Melting Point | 220.5 C (dec) |
| Molecular Weight | mol. wt. = 473.43 |
Resources and Citations
- For alum detection procedures see: N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology Archetype Publications, London, 2000, p. 34.
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water