Difference between revisions of "Castor oil"
(username removed) |
|||
| (8 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:image6_castoroil.jpg|thumb|Castor bean seeds and fruit]] | [[File:image6_castoroil.jpg|thumb|Castor bean seeds and fruit]] | ||
== Description == | == Description == | ||
| + | [[File:image5_castoroil.jpg|thumb|Castor Oil]] | ||
| + | A pale yellowish oil obtained from the seeds of the castor bean, ''Ricinus communis'', native to Africa but now growing in many temperate areas such as Brazil, India, Russia, and the U.S. Castor oil is a transparent, viscous liquid with a mild odor and an acrid taste. It contains ricinoleic acid (83-89%), [[oleic acid]] (3-6%), [[linoleic acid]] (4-7%), [[palmitic acid]] (1-2%), and [[stearic acid]] (1-2%) (Serpico and White 2000). In very thin layers, castor oil takes over a month to dry and thick layers never completely dry. Castor oil has been used as a lamp oil, lubricant, paint plasticizer, soap ingredient, and as an emollient to keep leather supple in low humidity areas. Dehydrated castor oil dries to a flexible but slightly tacky film. It is nonyellowing, water-resistant, and is used in [[alkyd resin|alkyd paints]] and varnishes. Castor oil is treated with [[sulfuric acid]] to make [[Turkey red oil]], an [[anionic detergent|anionic surfactant]] that has long been used as a dyeing aid. | ||
| − | |||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Ricinus communis; castor-oil; ricinus oil; oil of Palma Christi; tangantangan oil; noloid; ricinusolie (Dan.); Wunderbaum (Deut.); huile de ricin (Fr.); olio di ricino (It.) | Ricinus communis; castor-oil; ricinus oil; oil of Palma Christi; tangantangan oil; noloid; ricinusolie (Dan.); Wunderbaum (Deut.); huile de ricin (Fr.); olio di ricino (It.) | ||
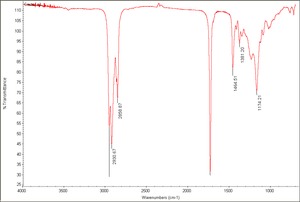
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Castor oil.TIF~FTIR(MFA)]]] |
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. Flash point = 229 C (444 F) | ||
| + | * Leaves and seeds are toxic. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC397280010&productDescription=CASTOR+OIL%2C+ETHOXYLATED%2C+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Miscible with ethanol, benzene, chloroform, carbon disulfide. Insoluble in water. Saponification value 176-187; Iodine value 81-91 | Miscible with ethanol, benzene, chloroform, carbon disulfide. Insoluble in water. Saponification value 176-187; Iodine value 81-91 | ||
| Line 21: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -10 to -18 | + | | -10 to -18 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.945-0.965 | + | | 0.945-0.965 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 30: | Line 34: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M.Serpico, R.White, "Oil, fat and wax" in ''Ancient Egyptian Materials and Technology'', P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 390-429. | |
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 Comment: melting point = -10 to -18C |
| − | * | + | * M. Doerner, ''The Materials of the Artist'', Harcourt, Brace & Co., 1934 |
| − | * | + | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 |
* ''Ancient Egyptian Materials and Technologies'', Paul Nicholson, Ian Shaw (eds.), Cambridge University Press, Cambridge, 2000 Comment: M.Serpico, R.White, "Oil, fat and wax" | * ''Ancient Egyptian Materials and Technologies'', Paul Nicholson, Ian Shaw (eds.), Cambridge University Press, Cambridge, 2000 Comment: M.Serpico, R.White, "Oil, fat and wax" | ||
| − | * | + | * Guy Weismantel, ''Paint Handbook'', McGraw-Hill Book Company, New York, 1981 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Castor_oil (Accessed Jan. 6 2006) |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 159 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1946 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1946 | ||
| Line 74: | Line 68: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Castor Oil." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Castor Oil." (Accessed 14 Apr. 2004 ). |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
Latest revision as of 07:28, 24 May 2022
Description
A pale yellowish oil obtained from the seeds of the castor bean, Ricinus communis, native to Africa but now growing in many temperate areas such as Brazil, India, Russia, and the U.S. Castor oil is a transparent, viscous liquid with a mild odor and an acrid taste. It contains ricinoleic acid (83-89%), Oleic acid (3-6%), Linoleic acid (4-7%), Palmitic acid (1-2%), and Stearic acid (1-2%) (Serpico and White 2000). In very thin layers, castor oil takes over a month to dry and thick layers never completely dry. Castor oil has been used as a lamp oil, lubricant, paint plasticizer, soap ingredient, and as an emollient to keep leather supple in low humidity areas. Dehydrated castor oil dries to a flexible but slightly tacky film. It is nonyellowing, water-resistant, and is used in alkyd paints and varnishes. Castor oil is treated with Sulfuric acid to make Turkey red oil, an anionic surfactant that has long been used as a dyeing aid.
Synonyms and Related Terms
Ricinus communis; castor-oil; ricinus oil; oil of Palma Christi; tangantangan oil; noloid; ricinusolie (Dan.); Wunderbaum (Deut.); huile de ricin (Fr.); olio di ricino (It.)
Risks
- Combustible. Flash point = 229 C (444 F)
- Leaves and seeds are toxic.
- ThermoFisher: SDS
Physical and Chemical Properties
Miscible with ethanol, benzene, chloroform, carbon disulfide. Insoluble in water. Saponification value 176-187; Iodine value 81-91
| CAS | 8001-79-4 |
|---|---|
| Melting Point | -10 to -18 C |
| Density | 0.945-0.965 g/ml |
| Refractive Index | 1.473-1.477 |
Resources and Citations
- M.Serpico, R.White, "Oil, fat and wax" in Ancient Egyptian Materials and Technology, P.Nicholson, I.Shaw (eds.), Cambridge University Press, 2000, p. 390-429.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982 Comment: melting point = -10 to -18C
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Ancient Egyptian Materials and Technologies, Paul Nicholson, Ian Shaw (eds.), Cambridge University Press, Cambridge, 2000 Comment: M.Serpico, R.White, "Oil, fat and wax"
- Guy Weismantel, Paint Handbook, McGraw-Hill Book Company, New York, 1981
- Wikipedia: http://en.wikipedia.org/wiki/Castor_oil (Accessed Jan. 6 2006)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 159
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1946
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "Castor Oil." (Accessed 14 Apr. 2004 ).
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: mp= -18, density=0.961, ref. index=1.4770, iodine value = 85.5, saponification value=180.3