Difference between revisions of "Egg white"
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | The colorless proteinaceous fluid ([ | + | The colorless proteinaceous fluid ([[albumin]]) surrounding the egg yolk of bird [[egg|eggs]]. Egg white is approximately 85% [[water]] and 12% [[protein]] with small amounts of [[fat]], [[carbohydrate|carbohydrates]], and [[salt|salts]]. The distribution of major [[amino acid|amino acids]] is: [[glutamic acid]] (13.9%), aspartic acid (10.5%), [[leucine]] (10.3%), lysine (8.0%), valine (8.3%), arginine (6.8%), [[alanine]] (6.3%), isoleucine (6.2%) and serine (5.8%) with no measurable amounts of [[hydroxyproline]] (Mills and White 1994). This proteinaceous mixture is chemically known as albumin but has been commercially spelled as [[albumen]] when used in food and as a binder for photographic emulsions. Albumin forms an amorphous solid when dried that is soluble in water. However, egg white is heat sensitive and forms an insoluble irreversible mass when heated to temperatures above 60 degrees centigrade. Egg white was used as a paint medium, called [[glair]], in illuminated manuscripts. It was also used in gilding for [[bole]] and for [[shell gold]] or powdered gold applications. Albumen was as an [[emulsion]] medium in mid-19th century photographic and lithographic prints. Egg white is also used for baking, textile dyeing, clarification of wines, and as an adhesive, coating, and binder. Medicinally, it is used as an antidote for [[mercury]] poisoning. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
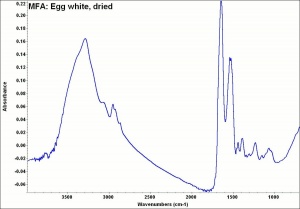
[[[SliderGallery rightalign|MFA- Egg white, dried.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Egg white, dried.jpg~FTIR]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in water after drying. Insoluble in water after heating. | Soluble in water after drying. Insoluble in water after heating. | ||
| − | == | + | == Resources and Citations == |
| − | J. | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: forms insoluble material at 70-75C |
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 22 | |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 Comment: becomes insoluble at about 75C |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * | + | * John S. Mills, Raymond White, ''The Organic Chemistry of Museum Objects'', Butterworth Heineman, London, 2nd ed., 1994 |
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 08:54, 19 August 2020
Description
The colorless proteinaceous fluid (Albumin) surrounding the egg yolk of bird eggs. Egg white is approximately 85% Water and 12% Protein with small amounts of Fat, carbohydrates, and salts. The distribution of major amino acids is: Glutamic acid (13.9%), aspartic acid (10.5%), Leucine (10.3%), lysine (8.0%), valine (8.3%), arginine (6.8%), Alanine (6.3%), isoleucine (6.2%) and serine (5.8%) with no measurable amounts of Hydroxyproline (Mills and White 1994). This proteinaceous mixture is chemically known as albumin but has been commercially spelled as Albumen when used in food and as a binder for photographic emulsions. Albumin forms an amorphous solid when dried that is soluble in water. However, egg white is heat sensitive and forms an insoluble irreversible mass when heated to temperatures above 60 degrees centigrade. Egg white was used as a paint medium, called Glair, in illuminated manuscripts. It was also used in gilding for Bole and for Shell gold or powdered gold applications. Albumen was as an Emulsion medium in mid-19th century photographic and lithographic prints. Egg white is also used for baking, textile dyeing, clarification of wines, and as an adhesive, coating, and binder. Medicinally, it is used as an antidote for Mercury poisoning.
Synonyms and Related Terms
albumin; albumen; glair
Physical and Chemical Properties
Soluble in water after drying. Insoluble in water after heating.
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: forms insoluble material at 70-75C
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 22
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986 Comment: becomes insoluble at about 75C
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994