Difference between revisions of "Carbon"
(username removed) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
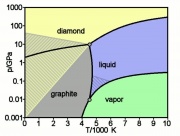
| − | A nonmetallic element that is contained in all organic molecules. Carbon has an abundance in the earth's crust of 0.027% where it occurs as [ | + | A nonmetallic element that is contained in all organic molecules. Carbon has an abundance in the earth's crust of 0.027% where it occurs as [[diamond|Diamonds]], [[graphite]], [[coal]] and as inorganic carbonates. Diamond is a valuable gemstone and is one of the hardest substance known. Graphite, although also composed of carbon, is a very soft, greasy substance. It is used in pencils, inks and as a lubricant. [[Carbon black]] is a principal black pigment. It is obtained by burning many different types of organic materials, such as [[acetylene black|Acetylene]], [[charcoal black|Wood]], [[fruit stone black|Fruit pits]], [[vine black|Vine stalks]], [[bone black|Bone]], [[ivory black|Ivory]], [[gas black|Gas]], [[cork black|Cork]], [[lampblack|Resins]], or [[oil black|Oils]]. Carbon is used in industry to manufacture fibers, make electrodes and as a sorbent and fill material. Carbon fibers are insoluble and can withstand high temperatures. Carbon arc electrodes can produce a light spectrum similar to sunlight. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
C; Koolstof (Ned.); carbone (Fr.); Kohlenstoff (Deut.); carbonio (It.); Carbono (Port.); carbono (Esp.); Kol (Sven.) | C; Koolstof (Ned.); carbone (Fr.); Kohlenstoff (Deut.); carbonio (It.); Carbono (Port.); carbono (Esp.); Kol (Sven.) | ||
| + | == Risks == | ||
| + | Nontoxic. | ||
| + | == Physical and Chemical Properties == | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 17: | Line 20: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | ~3550 | + | | ~3550 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.8-3.5 | + | | 1.8-3.5 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 26: | Line 29: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 4825 | + | | 4825 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | Web Elements: [http://www.webelements.com/webelements/elements/text/C/key.html Website] | + | * Web Elements: [http://www.webelements.com/webelements/elements/text/C/key.html Website] |
| − | + | * J.Winter, "The Characterization of Pigments Based on Carbon" Studies in Conservation, 28:49-66, 1983. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.139 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.139 | ||
| Line 49: | Line 48: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1855 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1855 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Carbon (Accessed Jan. 6, 2006) |
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Latest revision as of 15:08, 19 May 2022
Description
A nonmetallic element that is contained in all organic molecules. Carbon has an abundance in the earth's crust of 0.027% where it occurs as Diamonds, Graphite, Coal and as inorganic carbonates. Diamond is a valuable gemstone and is one of the hardest substance known. Graphite, although also composed of carbon, is a very soft, greasy substance. It is used in pencils, inks and as a lubricant. Carbon black is a principal black pigment. It is obtained by burning many different types of organic materials, such as Acetylene, Wood, Fruit pits, Vine stalks, Bone, Ivory, Gas, Cork, Resins, or Oils. Carbon is used in industry to manufacture fibers, make electrodes and as a sorbent and fill material. Carbon fibers are insoluble and can withstand high temperatures. Carbon arc electrodes can produce a light spectrum similar to sunlight.
Synonyms and Related Terms
C; Koolstof (Ned.); carbone (Fr.); Kohlenstoff (Deut.); carbonio (It.); Carbono (Port.); carbono (Esp.); Kol (Sven.)
Risks
Nontoxic.
Physical and Chemical Properties
| Composition | C (atomic no. 6) |
|---|---|
| CAS | 7440-44-0 |
| Melting Point | ~3550 C |
| Density | 1.8-3.5 g/ml |
| Molecular Weight | atomic wt = 12.0107 |
| Boiling Point | 4825 C |
Resources and Citations
- Web Elements: Website
- J.Winter, "The Characterization of Pigments Based on Carbon" Studies in Conservation, 28:49-66, 1983.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.139
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1855
- Wikipedia: http://en.wikipedia.org/wiki/Carbon (Accessed Jan. 6, 2006)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998