Difference between revisions of "Vermiculite"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in [ | + | A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in [[plaster|plaster]], [[concrete|concrete]], [[brick|brick]], [[rubber|rubber]], [[soil|soil]], [[paper|paper]], [[paint|paint]], and [[plastic|plastics]]. |
| − | |||
[[File:vermiculite2.jpg|thumb|Vermiculite, expanded]] | [[File:vermiculite2.jpg|thumb|Vermiculite, expanded]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
hydrated magnesium aluminum iron silicate; exfoliated hydrobiotite; Zonolite insulation; Microfil; Microlite; Verxite | hydrated magnesium aluminum iron silicate; exfoliated hydrobiotite; Zonolite insulation; Microfil; Microlite; Verxite | ||
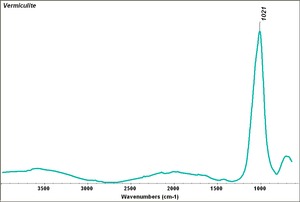
| + | [[[SliderGallery rightalign|Vermiculite.TIF~FTIR (MFA)]]] | ||
| − | + | == Risks == | |
| − | + | * Vermiculite mined prior to 1990 may contain asbestos which is toxic by ingestion and inhalation. | |
| − | == | + | * Noncombustible. |
| − | + | * Resistant to insects, bacteria, and fungi. | |
| − | + | * Millipore Sigma: [https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=Z765422&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fz765422%3Flang%3Den SDS] | |
| − | + | ==Physical and Chemical Properties== | |
| − | Fracture = | + | * Unaffected by water, acids, alkalis or organic solvents. |
| + | * Can expand 6-20 times when heated. Expanded vermiculite can absorb 200-500% of its weight in liquid. | ||
| + | * Fracture = uneven | ||
| + | * Crystal system = monoclinic | ||
| + | * Cleavage = perfect | ||
| + | * Streak = pale yellow | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 33: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.04-0.15 (expanded) | + | | 0.04-0.15 g/ml (expanded) |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Vermiculite." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Vermiculite." (Accessed 16 Mar. 2004). |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Vermiculite (Accessed Sept. 20, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 57: | Line 54: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London | + | * Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London. |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:24, 25 June 2022
Description
A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in Plaster, Concrete, Brick, Rubber, Soil, Paper, Paint, and plastics.
Synonyms and Related Terms
hydrated magnesium aluminum iron silicate; exfoliated hydrobiotite; Zonolite insulation; Microfil; Microlite; Verxite
Risks
- Vermiculite mined prior to 1990 may contain asbestos which is toxic by ingestion and inhalation.
- Noncombustible.
- Resistant to insects, bacteria, and fungi.
- Millipore Sigma: SDS
Physical and Chemical Properties
- Unaffected by water, acids, alkalis or organic solvents.
- Can expand 6-20 times when heated. Expanded vermiculite can absorb 200-500% of its weight in liquid.
- Fracture = uneven
- Crystal system = monoclinic
- Cleavage = perfect
- Streak = pale yellow
| CAS | 1318-00-9 |
|---|---|
| Mohs Hardness | 2-3 |
| Density | 0.04-0.15 g/ml (expanded) |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095
- Encyclopedia Britannica, http://www.britannica.com Comment: Vermiculite." (Accessed 16 Mar. 2004).
- Wikipedia: http://en.wikipedia.org/wiki/Vermiculite (Accessed Sept. 20, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London.