Difference between revisions of "Copper resinate"
(username removed) |
|||
| (14 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | The common name for a transparent green glaze containing copper salts of resin acids. Examples of copper resinate layers have been seen on manuscripts dating to the 8th century but it was most commonly used in 15th - 17th c. paintings. Several recipes for copper resinate indicate it is a mixture of [ | + | The common name for a transparent green glaze containing copper salts of resin acids. Examples of copper resinate layers have been seen on manuscripts dating to the 8th century but it was most commonly used in 15th - 17th c. paintings. Several recipes for copper resinate indicate it is a mixture of [[verdigris]] in [[Venice turpentine]] while others describe it as a mixture of verdigris in an oil/resin medium. Current preparation techniques melt natural resins then mix in reactive copper salts such as [[copper acetate]], [[copper hydroxide]], [[Copper oxide red|copper oxide]], or [[basic copper carbonate]] (Kuhn 1993). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
cupric resinate; transparent copper green; Kupferresinat (Deut.); résinate de cuivre (Fr.); resinato de cobre (Esp., Port.); resinato di rame (It.) | cupric resinate; transparent copper green; Kupferresinat (Deut.); résinate de cuivre (Fr.); resinato de cobre (Esp., Port.); resinato di rame (It.) | ||
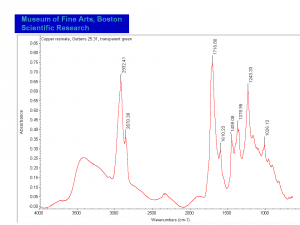
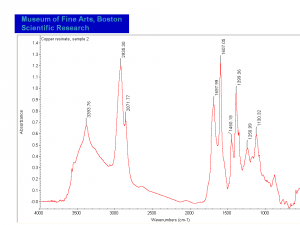
| − | [[[SliderGallery rightalign|MFA | + | [[[SliderGallery rightalign|Copper Resinate.PNG~FTIR (MFA)|Copper Resinate, sample 2.PNG~FTIR (MFA)]]] |
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Decomposes with heat. | ||
| + | * Turns brown with exposure to short-wave ultraviolet light. | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in ether, oils, and many organic solvents (benzene, chloroform, mineral spirits, etc.). Insoluble in water. | Soluble in ether, oils, and many organic solvents (benzene, chloroform, mineral spirits, etc.). Insoluble in water. | ||
| Line 21: | Line 27: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * H. Kuhn, "Verdigris and Copper Resinate", in ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. | |
| − | + | * Pigments Through the Ages: [http://www.webexhibits.org/pigments/indiv/overview/curesinate.html| Copper Resinate] | |
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: p. 110 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: p. 110 | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
| − | |||
* R. Newman, E. Farrell, 'House Paint Pigments', ''Paint in America '', R. Moss ed., Preservation Press, New York City, 1994 | * R. Newman, E. Farrell, 'House Paint Pigments', ''Paint in America '', R. Moss ed., Preservation Press, New York City, 1994 | ||
| Line 47: | Line 45: | ||
* Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:16, 4 July 2022
Description
The common name for a transparent green glaze containing copper salts of resin acids. Examples of copper resinate layers have been seen on manuscripts dating to the 8th century but it was most commonly used in 15th - 17th c. paintings. Several recipes for copper resinate indicate it is a mixture of Verdigris in Venice turpentine while others describe it as a mixture of verdigris in an oil/resin medium. Current preparation techniques melt natural resins then mix in reactive copper salts such as Copper acetate, Copper hydroxide, copper oxide, or Basic copper carbonate (Kuhn 1993).
Synonyms and Related Terms
cupric resinate; transparent copper green; Kupferresinat (Deut.); résinate de cuivre (Fr.); resinato de cobre (Esp., Port.); resinato di rame (It.)
Risks
- Combustible.
- Decomposes with heat.
- Turns brown with exposure to short-wave ultraviolet light.
Physical and Chemical Properties
Soluble in ether, oils, and many organic solvents (benzene, chloroform, mineral spirits, etc.). Insoluble in water.
Appears microscopically as irregular, green fragments.
| Refractive Index | 1.52 |
|---|
Resources and Citations
- H. Kuhn, "Verdigris and Copper Resinate", in Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- Pigments Through the Ages: Copper Resinate
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: p. 110
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000