Difference between revisions of "Verdigris"
| (21 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:422 verdigris.jpg|thumb|Verdigris]] | [[File:422 verdigris.jpg|thumb|Verdigris]] | ||
| − | == Description == | + | == Description == |
| + | [[File:verdigris C100x.jpg|thumb|Verdigris at 100x (visible light left; UV light right)]] | ||
| + | A dark bluish-green pigment composed of [[copper acetate, basic|basic copper acetate]]. Verdigris has been manufactured since ancient times by placing copper plates over vats of fermenting grape skins. The acetic acid quickly reacts to form basic copper acetate. When used directly as a pigment, verdigris can discolor from green to black in oil paints, fade in watercolor paints, and react with a paper support. Thus, it is more often used to make copper resinate and as a drier for linseed oil. Verdigris has also been used to dye fabrics and is still used as a colorant and fungicide in antifouling paints. | ||
| − | + | Historically, the various types of copper corrosion products were not differentiated but rather lumped together and called [[aerugo]] or verdigris. In the 20th century, this practice has carried over with the use of verdigris as a common, though chemically incorrect, name for natural green patinas formed on outdoor [[copper]], [[brass]], and [[bronze]]. Depending on atmospheric pollutants, however, these corrosion products are typically composed of [[copper sulfate]], [[copper chloride]], or [[basic copper carbonate]]. | |
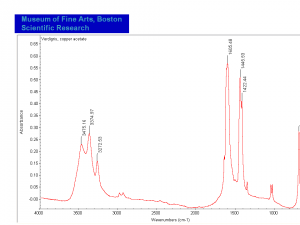
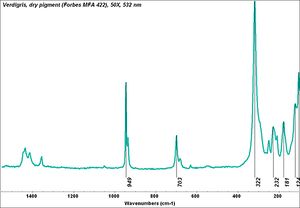
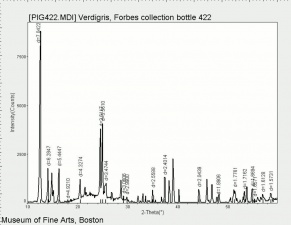
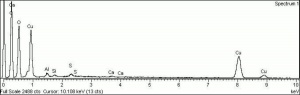
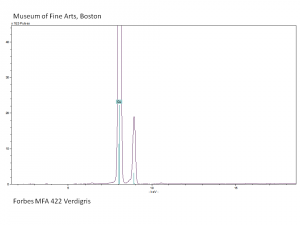
| − | + | [[[SliderGallery rightalign|Verdigris.PNG~FTIR (MFA)|Verdigris, dry pigment (Forbes MFA 422), 50X, 532 nm resize.jpg~Raman (MFA)|PIG422.jpg~XRD (MFA)|f422sem.jpg~SEM (MFA)|f422edsbw.jpg~EDS (MFA)|Slide29 FC422.PNG~XRF (MFA)]]] | |
| − | |||
| − | |||
| − | [[ | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | basic copper acetate; Pigment Green 20; vert-de-gris (Fr.); Verdigris (Deut.); Grünspan (Deut.); verdigris (It., Ned., Port.); verderame (It.); cardenillo (Esp.), verdete (Esp.); aerugo (Lat.); viride aeris (Lat.); aeruca; zangar; Spanish green; Van Eyck green; copper green; copper rust; copper subacetate; Montpelier green | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | == Risks == | |
| − | == | + | * Toxic by ingestion. |
| + | * Turns brown or black in contact with sulfur containing compounds. | ||
| + | * Decomposes with heat to produce acetic acid fumes and black residue. | ||
| + | ==Physical and Chemical Properties== | ||
| − | + | * Pleochroic changing from pale green to dark blue. | |
| − | + | * Strongly birefringent. (with the exception of copper resinates) | |
| − | == | + | * Tabular crystals with rhombic and hexagonal faces. |
| + | * Soluble in acids. Slightly soluble in water. | ||
| + | * Composition = Cu(C2H3O2)2-2Cu(OH)2 | ||
| + | * Refractive Index = 1.53; 1.56 | ||
| + | ==Additional Images== | ||
<gallery> | <gallery> | ||
| − | File:33_Verdigris_200X.jpg|Verdigris | + | File:33_Verdigris_200X.jpg|Verdigris at 200x |
| − | File:33_Verdigris_200X_SC.jpg|Verdigris | + | File:33_Verdigris_200X_SC.jpg|Verdigris at 200x slightly crossed polars |
| − | File:33_Verdigris_200X_pol.jpg|Verdigris | + | File:33_Verdigris_200X_pol.jpg|Verdigris at 200x polarized light |
| + | File:EV 106 VIS 100x annotated.jpg|Verdigris paint layer in architectural paint cross-section, Colonial Williamsburg Foundation, Thomas Everard House (1719) | ||
| + | File:RE 57c VIS 200x_with arrow.jpg|Verdigris paint layer in architectural paint cross-section, Colonial Williamsburg Foundation, George Reid House (mid 18th c.) | ||
</gallery> | </gallery> | ||
| − | + | ==Resources and Citations== | |
| − | == | + | * H. Kuhn, "Verdigris and Copper Resinate", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. |
| − | |||
| − | * ''Artists | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment' | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment' | ||
| − | |||
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | |
| − | * Art and Architecture Thesaurus Online, | + | * Pigments Through the Ages - http://webexhibits.org/pigments/indiv/history/verdigris.html - alpha = 1.53; gamma = 1.56 |
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Verdigris Verdigris] Accessed March 2025 | |
| − | * | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:27, 20 March 2025
Description
A dark bluish-green pigment composed of basic copper acetate. Verdigris has been manufactured since ancient times by placing copper plates over vats of fermenting grape skins. The acetic acid quickly reacts to form basic copper acetate. When used directly as a pigment, verdigris can discolor from green to black in oil paints, fade in watercolor paints, and react with a paper support. Thus, it is more often used to make copper resinate and as a drier for linseed oil. Verdigris has also been used to dye fabrics and is still used as a colorant and fungicide in antifouling paints.
Historically, the various types of copper corrosion products were not differentiated but rather lumped together and called Aerugo or verdigris. In the 20th century, this practice has carried over with the use of verdigris as a common, though chemically incorrect, name for natural green patinas formed on outdoor Copper, Brass, and Bronze. Depending on atmospheric pollutants, however, these corrosion products are typically composed of Copper sulfate, Copper chloride, or Basic copper carbonate.
Synonyms and Related Terms
basic copper acetate; Pigment Green 20; vert-de-gris (Fr.); Verdigris (Deut.); Grünspan (Deut.); verdigris (It., Ned., Port.); verderame (It.); cardenillo (Esp.), verdete (Esp.); aerugo (Lat.); viride aeris (Lat.); aeruca; zangar; Spanish green; Van Eyck green; copper green; copper rust; copper subacetate; Montpelier green
Risks
- Toxic by ingestion.
- Turns brown or black in contact with sulfur containing compounds.
- Decomposes with heat to produce acetic acid fumes and black residue.
Physical and Chemical Properties
- Pleochroic changing from pale green to dark blue.
- Strongly birefringent. (with the exception of copper resinates)
- Tabular crystals with rhombic and hexagonal faces.
- Soluble in acids. Slightly soluble in water.
- Composition = Cu(C2H3O2)2-2Cu(OH)2
- Refractive Index = 1.53; 1.56
Additional Images
Resources and Citations
- H. Kuhn, "Verdigris and Copper Resinate", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment'
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments Through the Ages - http://webexhibits.org/pigments/indiv/history/verdigris.html - alpha = 1.53; gamma = 1.56
- Wikipedia: Verdigris Accessed March 2025