Copper chloride
Jump to navigation
Jump to search
Description
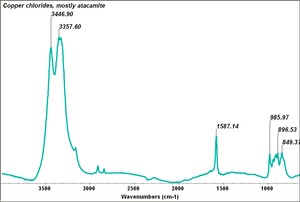
Several forms of copper chloride are found as minerals: including Atacamite, Paratacamite, eriochalcite, tolbachite, and nantokite. Pure anhydrous copper chloride is composed of dark yellow crystals that absorb water to form green deliquescent crystals. Copper chloride is used as a Disinfectant, Fungicide, and Wood preservative. It is also used as a fixer in photography, a Mordant in dyeing textiles and a component in indelible inks. In metallurgy, copper chloride is used refine Gold and Silver, to recover Mercury from ore, and to electroplate Copper on Aluminum. Copper chloride is also used as a Glass and ceramics pigment.
Synonyms and Related Terms
cupric chloride; copper (II) chloride
Risks
- Toxic by ingestion and inhalation.
- Skin contact causes irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
- Composition = CuCl2 (mol. wt. = 134.45)
- CAS = 7447-39-4
- Density = 2.54 g/ml
- Molecular Weight
- Refractive Index = 1.644, 1.684, 1.742
- Soluble in alcohols, water and acetone. Aqueous solution is acidic.
Resources and Citations
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2699
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.644, 1.684, 1.742