Ammonium chloride: Difference between revisions
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
No edit summary |
||
| Line 2: | Line 2: | ||
Slightly hygroscopic white crystals historically used to separate [[gold|gold]] from [[silver|silver]] or [[copper|copper]]. Ammonium chloride was known in ancient times where legend tells it was observed in the Temple of Zeus-Ammon in Egypt as it was scraped from the ceiling after camel dung was burned. Ammoniun chloride has also been used as a [[mordant|mordant]], as a textile finishing agent to produce luster and as a tanning agent for [[leather|leather]]. Currently, the most common uses for ammonium chloride are as a soldering flux for [[iron|iron]], as a component in galvanizing solutions, as an electrolyte in dry cells and as a metal coloring agent. | Slightly hygroscopic white crystals historically used to separate [[gold|gold]] from [[silver|silver]] or [[copper|copper]]. Ammonium chloride was known in ancient times where legend tells it was observed in the Temple of Zeus-Ammon in Egypt as it was scraped from the ceiling after camel dung was burned. Ammoniun chloride has also been used as a [[mordant|mordant]], as a textile finishing agent to produce luster and as a tanning agent for [[leather|leather]]. Currently, the most common uses for ammonium chloride are as a soldering flux for [[iron|iron]], as a component in galvanizing solutions, as an electrolyte in dry cells and as a metal coloring agent. | ||
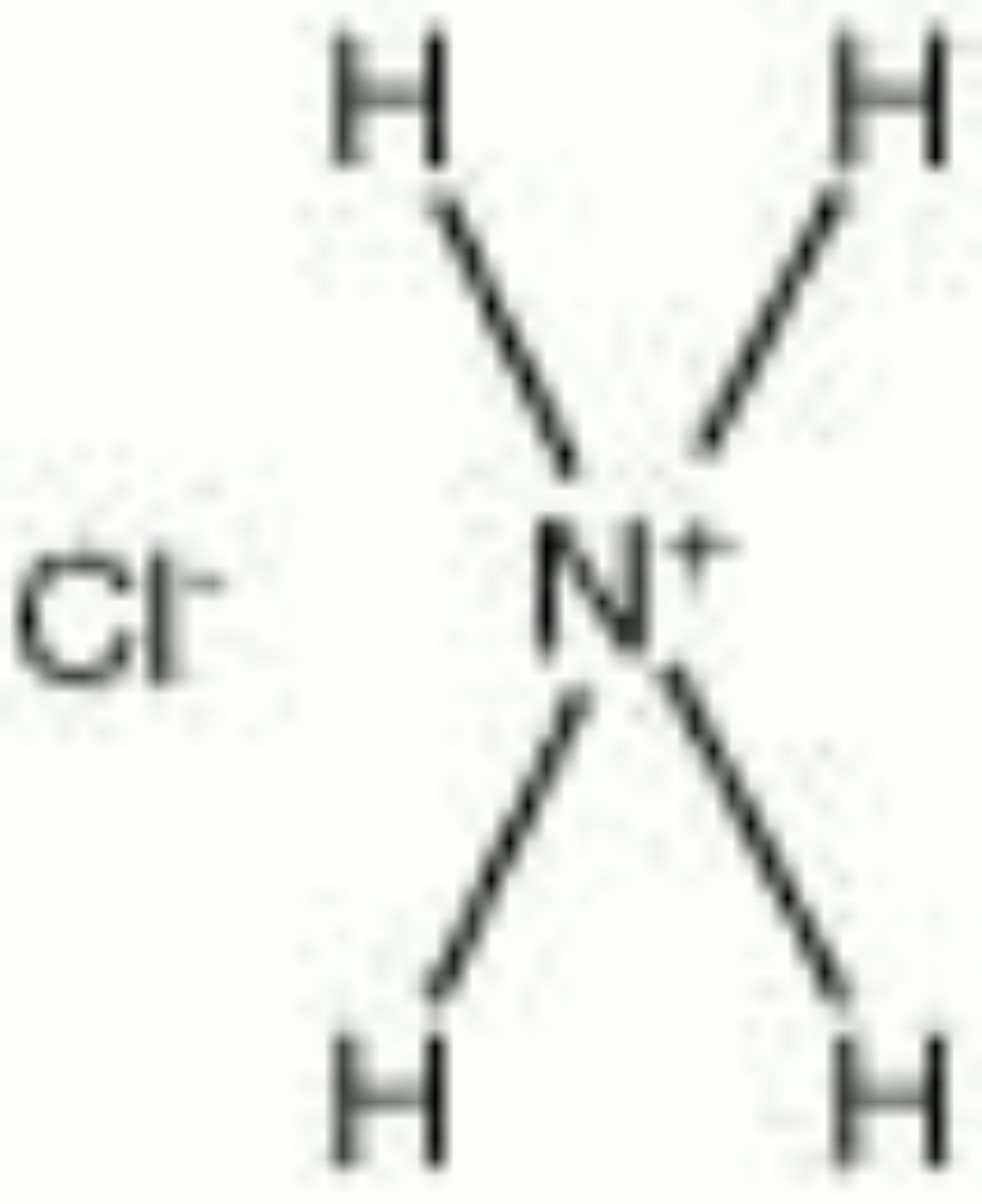
[[[SliderGallery rightalign|ammonium chloride.jpg~Chemical structure]]] | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sal ammonia; sal ammoniac; sal ammoniacum; ammonium muriate; salmiak (Ces.); chlorid amonný (Ces.); ammoniumklorid (Dan., Sven.); Ammoniumchlorid (Deut.); cloruro di ammonio (It.); salmiakzout (Ned.); chlorek amonu (Pol.); nashadir (Arab.); naosha (Chin.); nao sadar (India); salmiac; Amchlor; Darammon | sal ammonia; sal ammoniac; sal ammoniacum; ammonium muriate; salmiak (Ces.); chlorid amonný (Ces.); ammoniumklorid (Dan., Sven.); Ammoniumchlorid (Deut.); cloruro di ammonio (It.); salmiakzout (Ned.); chlorek amonu (Pol.); nashadir (Arab.); naosha (Chin.); nao sadar (India); salmiac; Amchlor; Darammon | ||
== | == Risks == | ||
* Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25168C.pdf SDS] | |||
== Physical and Chemical Properties == | |||
Crystals form aggregates with conchoidal fracture. | * Soluble in water, methanol, ethanol. Insoluble in acetone, ether, ethyl acetate. | ||
* Incompatible with alkalis and lead or silver salts. | |||
* Crystals form aggregates with conchoidal fracture. | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 27: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| 340-350 (sublimes) | | 340-350 C (sublimes) | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| 1.5274 | | 1.5274 g/ml | ||
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 39: | Line 41: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| 520 | | 520 C | ||
|} | |} | ||
== | ==Resources and Citations== | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 60: | Line 58: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Ammonium chloride" | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Ammonium chloride" [Accessed 25 Jan. 2003]. | ||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| Line 66: | Line 64: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
* Wikipedia | * Wikipedia: http://en.wikipedia.org/wiki/Ammonium_chloride (Accessed Mar. 20, 2006) -for non-English terms | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
Latest revision as of 13:47, 26 April 2022
Description
Slightly hygroscopic white crystals historically used to separate gold from silver or copper. Ammonium chloride was known in ancient times where legend tells it was observed in the Temple of Zeus-Ammon in Egypt as it was scraped from the ceiling after camel dung was burned. Ammoniun chloride has also been used as a mordant, as a textile finishing agent to produce luster and as a tanning agent for leather. Currently, the most common uses for ammonium chloride are as a soldering flux for iron, as a component in galvanizing solutions, as an electrolyte in dry cells and as a metal coloring agent.
Synonyms and Related Terms
sal ammonia; sal ammoniac; sal ammoniacum; ammonium muriate; salmiak (Ces.); chlorid amonný (Ces.); ammoniumklorid (Dan., Sven.); Ammoniumchlorid (Deut.); cloruro di ammonio (It.); salmiakzout (Ned.); chlorek amonu (Pol.); nashadir (Arab.); naosha (Chin.); nao sadar (India); salmiac; Amchlor; Darammon
Risks
- Fisher Scientific: SDS
Physical and Chemical Properties
- Soluble in water, methanol, ethanol. Insoluble in acetone, ether, ethyl acetate.
- Incompatible with alkalis and lead or silver salts.
- Crystals form aggregates with conchoidal fracture.
| Composition | NH4Cl |
|---|---|
| CAS | 12125-02-9 |
| Mohs Hardness | 1.0 - 2.0 |
| Melting Point | 340-350 C (sublimes) |
| Density | 1.5274 g/ml |
| Molecular Weight | mol. wt. = 53.5 |
| Refractive Index | 1.639 |
| Boiling Point | 520 C |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 58
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Encyclopedia Britannica, http://www.britannica.com Comment: "Ammonium chloride" [Accessed 25 Jan. 2003].
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Wikipedia: http://en.wikipedia.org/wiki/Ammonium_chloride (Accessed Mar. 20, 2006) -for non-English terms
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985