Difference between revisions of "Ferric sulfate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
(→Risks) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Grayish-white powder formed by adding [[sulfuric acid]] to [[ferric hydroxide]]. Ferric sulfate is very [[hygroscopic]]. It is used as a mordant in textile dyeing and as a component in [[iron gall ink|iron gall inks]]. Ferric sulfate is also used in water purification systems. Ferric sulfate occurs naturally in minerals with varying states of hydration, including lausenite [Fe2(SO4)3- | + | Grayish-white powder formed by adding [[sulfuric acid]] to [[ferric hydroxide]]. Ferric sulfate is very [[hygroscopic]]. It is used as a mordant in textile dyeing and as a component in [[iron gall ink|iron gall inks]]. Ferric sulfate is also used in water purification systems. Ferric sulfate occurs naturally in minerals with varying states of hydration, including lausenite [Fe2(SO4)3-5H2O], [[kornelite]] [Fe2(SO4)3-7H2O], [[coquimbite]] [Fe2(SO4)3-9H2O], and [[quenstedtite]] [Fe2(SO4)3-10H2O]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate | + | iron III sulfate; ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate |
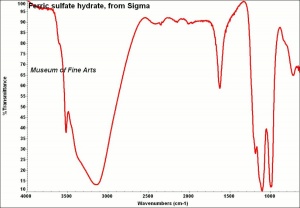
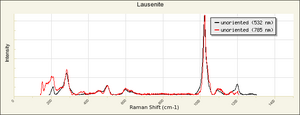
| − | [[[SliderGallery rightalign|FerricsulfateSigmaIR.jpg~FTIR]]] | + | [[[SliderGallery rightalign|FerricsulfateSigmaIR.jpg~FTIR|Lausenite Raman RRUFF X070004.png~Raman (U of Arizona)]]] |
| − | == | + | == Risks == |
| + | |||
| + | * Non-combustible. | ||
| + | * Decomposes with light | ||
| + | * Respiratory system irritant | ||
| + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-f/S25322A.pdf SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties=== | ||
Slightly soluble in water and alcohol. Insoluble in organic solvents. | Slightly soluble in water and alcohol. Insoluble in organic solvents. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 480 (d) | + | | 480 C (d) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.0-2.1 | + | | 2.0-2.1 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 10:51, 9 December 2022
Description
Grayish-white powder formed by adding Sulfuric acid to Ferric hydroxide. Ferric sulfate is very Hygroscopic. It is used as a mordant in textile dyeing and as a component in iron gall inks. Ferric sulfate is also used in water purification systems. Ferric sulfate occurs naturally in minerals with varying states of hydration, including lausenite [Fe2(SO4)3-5H2O], Kornelite [Fe2(SO4)3-7H2O], Coquimbite [Fe2(SO4)3-9H2O], and Quenstedtite [Fe2(SO4)3-10H2O].
Synonyms and Related Terms
iron III sulfate; ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate
Risks
- Non-combustible.
- Decomposes with light
- Respiratory system irritant
- Fisher Scientific: SDS
Physical and Chemical Properties=
Slightly soluble in water and alcohol. Insoluble in organic solvents.
| Composition | Fe2(SO4)3 |
|---|---|
| CAS | 10028-22-5 |
| Melting Point | 480 C (d) |
| Density | 2.0-2.1 g/ml |
| Molecular Weight | 399.88 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3963