Difference between revisions of "Methyl acetate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 2: | Line 2: | ||
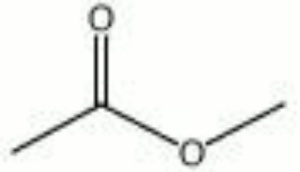
A colorless solvent with a fragrant odor. Methyl acetate is the methyl ester of acetic acid. It is used as a solvent for [[cellulose%20nitrate|cellulose nitrate]] and as a [[paint%20remover|paint remover]]. It is also used in the manufacture of artificial leather. | A colorless solvent with a fragrant odor. Methyl acetate is the methyl ester of acetic acid. It is used as a solvent for [[cellulose%20nitrate|cellulose nitrate]] and as a [[paint%20remover|paint remover]]. It is also used in the manufacture of artificial leather. | ||
| + | [[[SliderGallery rightalign|methyl acetate.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 8: | ||
methyl ester of acetic acid | methyl ester of acetic acid | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Overexposure can cause skin irritation. | ||
| + | * Ingestion may cause headaches, drowsiness and optical atrophy. | ||
| + | * May decompose in aqueous solutions to form acetic acid. | ||
| + | * Flammable. Flash point = -9C (15F). Fire and explosion risk. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC371831000&productDescription=METHYL+ACETATE&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water. Miscible in ethanol and ether. | Soluble in water. Miscible in ethanol and ether. | ||
| Line 24: | Line 31: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -98 | + | | -98 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9279 | + | | 0.9279 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 43: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 56.9 | + | | 56.9 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 53: | Line 52: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6089; ref. index=1.3619 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6089; ref. index=1.3619 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Methyl_acetate (Accessed Jan. 6 2006) |
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Latest revision as of 14:00, 18 October 2022
Description
A colorless solvent with a fragrant odor. Methyl acetate is the methyl ester of acetic acid. It is used as a solvent for Cellulose nitrate and as a Paint remover. It is also used in the manufacture of artificial leather.
Synonyms and Related Terms
methyl ester of acetic acid
Risks
- Overexposure can cause skin irritation.
- Ingestion may cause headaches, drowsiness and optical atrophy.
- May decompose in aqueous solutions to form acetic acid.
- Flammable. Flash point = -9C (15F). Fire and explosion risk.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Miscible in ethanol and ether.
Hydrolyzes in water to acetic acid.
| Composition | CH3COOCH3 |
|---|---|
| CAS | 79-20-9 |
| Melting Point | -98 C |
| Density | 0.9279 g/ml |
| Molecular Weight | mol. wt. = 74 |
| Refractive Index | 1.3619 |
| Boiling Point | 56.9 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6089; ref. index=1.3619
- Wikipedia: http://en.wikipedia.org/wiki/Methyl_acetate (Accessed Jan. 6 2006)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.360