Difference between revisions of "Rhodamine B"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (11 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[File:44 rhodamine.jpg|thumb|Rhodamine]] | [[File:44 rhodamine.jpg|thumb|Rhodamine]] | ||
== Description == | == Description == | ||
| − | + | [[File:rhodamine C100x.jpg|thumb|rhodamine at 100x]] | |
A strong, bright red [[fluorescent%20dye|fluorescent dye]]. Rhodamine B is a [[basic%20dye|basic dye]] that was developed in 1887 by Ceresole. It is used as [[textile|textile]] and [[paper|paper]] dye, as a pigment, and as a staining reagent for the detection of [[fat|fats]] and [[oil|oils]]. Some oil modified materials such as [[alkyd%20resin|alkyds]] and [[polyurethane|urethanes]] will also stain with rhodamine (Wolbers et al 1990). Rhodamine B has been used as a fluorescent colorant in [[ink|inks]] ([[ballpoint%20ink|ballpoint]], [[printing%20ink|printing]]), [[wood%20stain|wood stains]], [[distemper|distemper]] paints, and shoe polish. | A strong, bright red [[fluorescent%20dye|fluorescent dye]]. Rhodamine B is a [[basic%20dye|basic dye]] that was developed in 1887 by Ceresole. It is used as [[textile|textile]] and [[paper|paper]] dye, as a pigment, and as a staining reagent for the detection of [[fat|fats]] and [[oil|oils]]. Some oil modified materials such as [[alkyd%20resin|alkyds]] and [[polyurethane|urethanes]] will also stain with rhodamine (Wolbers et al 1990). Rhodamine B has been used as a fluorescent colorant in [[ink|inks]] ([[ballpoint%20ink|ballpoint]], [[printing%20ink|printing]]), [[wood%20stain|wood stains]], [[distemper|distemper]] paints, and shoe polish. | ||
| − | [[File: | + | [[File:Rhodamine B_abs.ems.jpg|thumb|Absorption and fluorescence emission spectra]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | Basic Violet 10; CI 45170; tetraethylrhodamine; D&C Red No. 19, Solvent Red 49; Pigment Violet 1 (phosphotungstomolybdic acid salt); Pigment Red 169 (copper ferrocyanide salt); Pigment Red 173 (aluminum salt); Rhodamine (Deut.); Rodamina B (Esp.); rodamina B (Port.); rhodamine B (Fr.) | + | Basic Violet 10; CI 45170; tetraethylrhodamine; D&C Red No. 19, Solvent Red 49; Pigment Violet 1 (phosphotungstomolybdic acid salt); Pigment Red 169 (copper ferrocyanide salt); Pigment Red 173 (aluminum salt); Rhodamine (Deut.); Rodamina B (Esp.); rodamina B (Port.); rhodamine B (Fr.) |
| + | |||
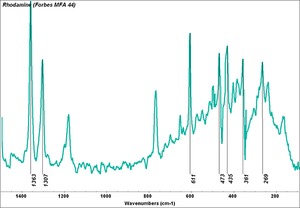
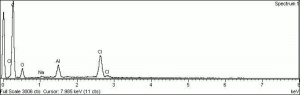
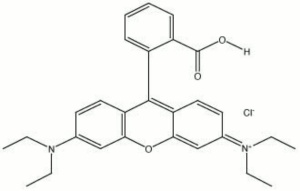
| + | [[[SliderGallery rightalign|PV001 sun rhodamine b 221-0035.TIF~FTIR 221-0035(MFA)|PV1 rhodamine b (magruder mm0107-dc).TIF~FTIR mm0107-dc(MFA)|Pv1 rhodamine B (magruder mm0122-dc).TIF~FTIR mm0122-dc(MFA)|Pv1 rhodamine B (magruder mm1219-dc).TIF~FTIR mm1219-dc(MFA)|Rhodamine (Forbes MFA 44) copy.tif~Raman (MFA)|f44sem.jpg~SEM|f44edsbw.jpg~EDS|rhodamine b.jpg~Chemical structure]]] | ||
| + | |||
| + | == Comparisons == | ||
| − | + | {| class="wikitable" | |
| + | ! Pigment number !! Manufacture !! Pigment name !! Manufacture CI number !! Comments | ||
| + | |- | ||
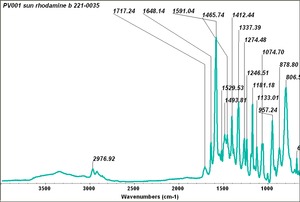
| + | | PV001 || Sun|| rhodamine B || 221-0035 || | ||
| + | |- | ||
| + | | PV001 || Magruder|| rhodamine B || mm0107-dc|| | ||
| + | |- | ||
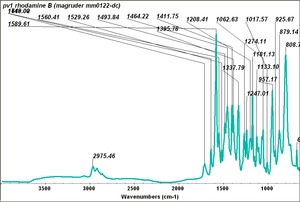
| + | | PV001 || Magruder || rhodamine B || mm0122-dc || | ||
| + | |- | ||
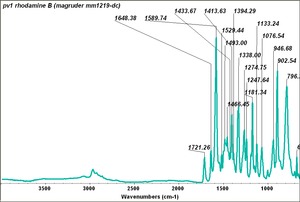
| + | | PV001 || Magruder || rhodamine B || mm1219-dc || | ||
| + | |- | ||
| + | |} | ||
| − | == | + | == Risks == |
| − | + | * Carcinogen. | |
| + | * Mutagen. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AAA1357218&productDescription=RHODAMINE+B+50G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | Maximum emission wavelength = 625 nm. | + | * Green crystals or violet powder. |
| + | * Soluble in water, ethanol, polar solvents. | ||
| + | * Maximum absorption wavelength = 545 nm. | ||
| + | * Maximum emission wavelength = 625 nm. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 48: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 210-211 | + | | 210-211 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 54: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * R. Wolbers, N. Sterman and C. Stavroudis, "Notes for Workshop on New Methods in the Cleaning of Paintings", 1990, GCI, Los Angeles. | ||
| − | + | * Website for absorption/fluorescent spectra: [http://www.omlc.ogi.edu/spectra/PhotochemCAD/html/rhodamineB.html http://www.omlc.ogi.edu/spectra/PhotochemCAD/html/rhodamineB.html] | |
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| Line 63: | Line 70: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8349 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8349 | ||
| − | * | + | * Dye History: www.straw.com/sig/dyehist - discovered 1887 |
* Aldrich Chemical Catalog Comment: p. 1299 | * Aldrich Chemical Catalog Comment: p. 1299 | ||
Latest revision as of 13:50, 25 August 2022
Description
A strong, bright red Fluorescent dye. Rhodamine B is a Basic dye that was developed in 1887 by Ceresole. It is used as Textile and Paper dye, as a pigment, and as a staining reagent for the detection of fats and oils. Some oil modified materials such as alkyds and urethanes will also stain with rhodamine (Wolbers et al 1990). Rhodamine B has been used as a fluorescent colorant in inks (ballpoint, printing), wood stains, Distemper paints, and shoe polish.
Synonyms and Related Terms
Basic Violet 10; CI 45170; tetraethylrhodamine; D&C Red No. 19, Solvent Red 49; Pigment Violet 1 (phosphotungstomolybdic acid salt); Pigment Red 169 (copper ferrocyanide salt); Pigment Red 173 (aluminum salt); Rhodamine (Deut.); Rodamina B (Esp.); rodamina B (Port.); rhodamine B (Fr.)
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
| PV001 | Sun | rhodamine B | 221-0035 | |
| PV001 | Magruder | rhodamine B | mm0107-dc | |
| PV001 | Magruder | rhodamine B | mm0122-dc | |
| PV001 | Magruder | rhodamine B | mm1219-dc |
Risks
- Carcinogen.
- Mutagen.
- ThermoFisher: SDS
Physical and Chemical Properties
- Green crystals or violet powder.
- Soluble in water, ethanol, polar solvents.
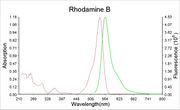
- Maximum absorption wavelength = 545 nm.
- Maximum emission wavelength = 625 nm.
| Composition | C28H31ClN2O3 |
|---|---|
| CAS | 81-88-9 |
| Melting Point | 210-211 C |
| Molecular Weight | mol. wt. = 478.68 |
Resources and Citations
- R. Wolbers, N. Sterman and C. Stavroudis, "Notes for Workshop on New Methods in the Cleaning of Paintings", 1990, GCI, Los Angeles.
- Website for absorption/fluorescent spectra: http://www.omlc.ogi.edu/spectra/PhotochemCAD/html/rhodamineB.html
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8349
- Dye History: www.straw.com/sig/dyehist - discovered 1887
- Aldrich Chemical Catalog Comment: p. 1299
- Colour Index International online at www.colour-index.org