Difference between revisions of "Arylide"
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A class of synthetic yellow, orange and red organic colorants. These insoluble direct azo dyes were first synthesized from aniline-based diazonium salts and acetoacetarylide in 1909. Arylide was sold commercially as Hansa yellow by Hoechst AG in 1915. Arylide yellows have good tinting strength, opacity and solvent resistance. They have good lightfastness and are primarily used in printing inks, plastic, rubbers, as well as architectural and artists paints. Monoarylide yellows have better lightfastness than diarylides. | + | A class of synthetic yellow, orange and red organic colorants. These insoluble direct azo dyes were first synthesized from aniline-based diazonium salts and acetoacetarylide in 1909. Arylide was sold commercially as [[Hansa yellow]] by Hoechst AG in 1915. Arylide yellows have good tinting strength, opacity and solvent resistance. They have good lightfastness and are primarily used in printing inks, plastic, rubbers, as well as architectural and artists paints. Monoarylide yellows have better lightfastness than [[Diarylide dye|diarylides]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 35: | Line 35: | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | Most arylide dyes are soluble in organic solvents. Resistant to water, oil, acids and bases. | + | Most arylide dyes are soluble in organic solvents. Resistant to water, oil, acids and bases. Melting Point = 150 (dec) |
| − | + | == Risks == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
May bleed in paints. Decomposes at temperatures over 150 C. | May bleed in paints. Decomposes at temperatures over 150 C. | ||
Latest revision as of 08:58, 30 October 2020
Description
A class of synthetic yellow, orange and red organic colorants. These insoluble direct azo dyes were first synthesized from aniline-based diazonium salts and acetoacetarylide in 1909. Arylide was sold commercially as Hansa yellow by Hoechst AG in 1915. Arylide yellows have good tinting strength, opacity and solvent resistance. They have good lightfastness and are primarily used in printing inks, plastic, rubbers, as well as architectural and artists paints. Monoarylide yellows have better lightfastness than diarylides.
Synonyms and Related Terms
monoarylide; diarylide; azo dye; monoazo dye; Hansa yellow; colorante azoico (Esp.); colorante azoico (It.)
Comparisons
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
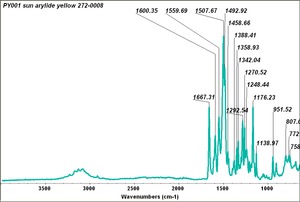
| PY001 | Sun | arylide yellow | 272-0008 | |
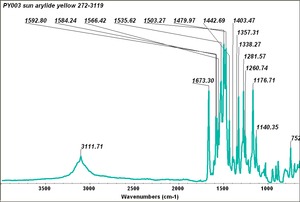
| PY003 | Sun | arylide yellow | 272-3119 | |
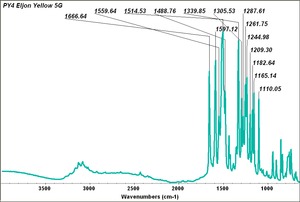
| PY004 | unknown | eljon yellow 5g | unknown | |
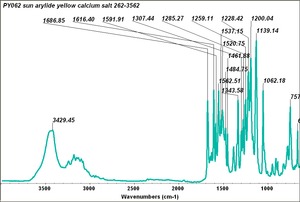
| PY062 | Sun | arylide yellow | 262-3562 | |
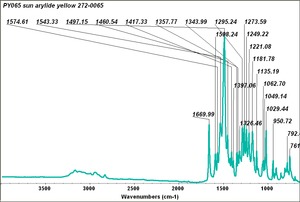
| PY065 | Sun | arylide yellow | 272-0065 | |
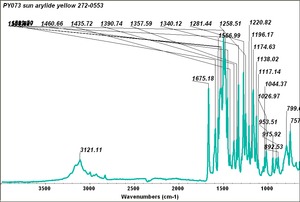
| PY073 | Sun | arylide yellow | 272-0553 | |
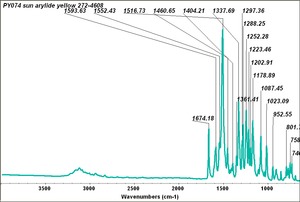
| PY074 | Sun | arylide yellow | 272-4608 | same spectrum for Kremer PY74 |
| PY074 | Kremer | unspecified | 23650 | |
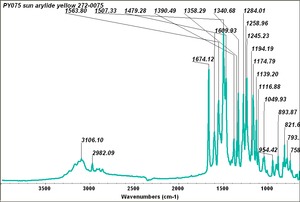
| PY075 | Sun | arylide yellow | 272-0075 |
Physical and Chemical Properties
Most arylide dyes are soluble in organic solvents. Resistant to water, oil, acids and bases. Melting Point = 150 (dec)
Risks
May bleed in paints. Decomposes at temperatures over 150 C.
Potential carcinogen.
Resources and Citations
- B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', Studies in the History of Art , National Gallery of Art, Washington DC, No. 57, 1997
- Website: www.handprint.com