Difference between revisions of "More on the data"
| (72 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:Colorsamples.jpg | + | [[File:Colorsamples.jpg|350px|right|Colorant samples]] |
| − | |||
For this research non-invasive, non-destructive techniques that do not require sampling from the prints were used to analyze the colorants. The analytical techniques used are X-ray Fluorescence (XRF), Excitation Emission Matrix (EEM), and Fiber Optic Reflectance (FORS) spectroscopies. Colorant samples were also formulated and printed using organic and inorganic materials traditionally believed to have been used during the Edo period (1603–1868). These were used to cross reference the analytical results. These methods, in conjunction with visual inspection, were then used to identify the colorants. | For this research non-invasive, non-destructive techniques that do not require sampling from the prints were used to analyze the colorants. The analytical techniques used are X-ray Fluorescence (XRF), Excitation Emission Matrix (EEM), and Fiber Optic Reflectance (FORS) spectroscopies. Colorant samples were also formulated and printed using organic and inorganic materials traditionally believed to have been used during the Edo period (1603–1868). These were used to cross reference the analytical results. These methods, in conjunction with visual inspection, were then used to identify the colorants. | ||
==Analytical Methods== | ==Analytical Methods== | ||
===What is XRF?=== | ===What is XRF?=== | ||
| − | [[X- | + | [[File:MFA XRF instrument.jpg|thumb|XRF analysis using an open architecture Bruker ARTAX]] |
| + | |||
| + | [[X-ray_fluorescence_(XRF) spectroscopy|X-ray fluorescence (XRF) spectroscopy]] is a nondestructive analytical method used to qualitatively and semi-quantitatively determine the elemental content of materials. The x-ray spectrum emitted from each element has a unique set of energies that are related to the type and number of atoms present in the sample. For this research, ARTAX open-architecture instrument was used. | ||
XRF is used to identify inorganic colorants based on their inorganic element composition. For example, orpiment can be identified using XRF because it can detect the arsenic in orpiment, which is an arsenic trisulfide. In combination with visual inspection (yellow color) it can be identified as orpiment because it is the only known yellow colorant with arsenic. | XRF is used to identify inorganic colorants based on their inorganic element composition. For example, orpiment can be identified using XRF because it can detect the arsenic in orpiment, which is an arsenic trisulfide. In combination with visual inspection (yellow color) it can be identified as orpiment because it is the only known yellow colorant with arsenic. | ||
| Line 12: | Line 13: | ||
===What is EEM?=== | ===What is EEM?=== | ||
| − | Excitation Emission Matrix (EEM) is a | + | [[File:MFA EEM.jpg|thumb|EEM analysis of colorants using an Agilent Cary Eclipse Spectrometer with a fiber optic probe]] |
| + | |||
| + | [[Excitation emission matrix (EEM)|Excitation Emission Matrix (EEM) spectroscopy]] is a method that results in a 3D pattern of the fluorescence of an organic material. The pattern is produced by plotting the intensity of a sample’s fluorescence emissions occurring as a series of stepped excitation wavelengths. Each material has a reproducible 3D fluorescence pattern that allows its identification. Even the lack of fluorescence, which indicates the material is non-fluorescent, also provides a key piece of information for identification of a colorant. The reference colorant samples were printed to create a set of baseline patterns for each colorant. EEM was used to identify some organic yellow and and most red colorants. For this research, an Agilent Cary Eclipse Spectrometer with a fiber optic probe was used. | ||
| + | {|style="float:left; margin-right: 10px;" | ||
| + | | [[File:Derrick Fluorescence reds max.jpg|150px|Excitation and emission maxima for safflower, madder, and sappanwood]] | ||
| + | |- | ||
| + | |<small>Excitation and emission maxima<br>of the three red colorants</small> | ||
| + | |- | ||
| + | |[[File:MFA EEM reds on print.jpg|150px|3-dimensional EEM spectra of three red colorants]] | ||
| + | |- | ||
| + | |<small>3D EEM plots of the three<br>red colorants</small> | ||
| + | |} | ||
| + | Excitation can occur at many wavelengths. When the instrument is set to scan through several excitation wavelengths, it can determine which wavelength excites the most molecules to fluoresce. This is called the Excitation maxima. Exposing the material to this maximum excitation wavelength, the emitted fluorescence or the emission curve can be measured and the point with the highest emission is called the Emission max. The Excitation and Emission maxima of the three reds, safflower, madder, and sappanwood are distinct (see left). Thus the three reds can be distinguished by their fluorescence measurements. The Emission maxima for safflower is in the yellow-orange region, madder is in the orange region, and sappanwood is in the orange-red region. | ||
| + | |||
| + | EEM has been easiest for identifying the reds when the spectral data is shown as a 3-dimensional plot, even in mixtures, where the excitation wavelength is given on the vertical axis and the emission wavelength on the horizontal access. The intensity of the absorption or emission is given by the color with dark red being the most intense. The angled bar on the plot is where the excitation and emission wavelengths are identical, thus the detector is saturated at these points. Shown on the left are the 3D EEM plots for safflower, madder, and sappanwood with their individually distinct patterns. | ||
| − | + | Additionally some of the yellow in ''ukiyo-e'' prints also fluoresce. For example, turmeric has a unique fluorescence or EEM pattern, making it an easily identifiable colorant using EEM. However, some of the other organic yellow colorants such as Japanese pagoda tree (''enju'') and gardenia (''kuchinashi'') which belong to a general chemical class known as flavonoids is difficult to reliably differentiate. | |
| + | <br> | ||
| + | <br> | ||
===What is FORS?=== | ===What is FORS?=== | ||
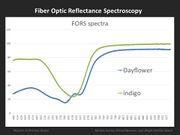
| − | Fiber | + | [[File:MFA FORS Blue spectra.jpg|thumb|FORS spectra for dayflower and indigo]] |
| + | [[Fiber optics reflectance spectroscopy (FORS)|Fiber Optics Reflectance spectroscopy (FORS)]] is a non-invasive technique that can be used for the examination of color as well as for the identification of colorants. FORS uses a fiber optic probe and a pulsed xenon source to provide good sensitivity, examine small areas, and minimize the interference from ambient light. For this research, an Ocean Optics miniature spectrometer was used. | ||
| − | + | FORS is able to distinguish between the three common blue colorants used in Japanese woodblock prints: dayflower, indigo, and Prussian blue. This can be accomplished, not only in blue printed areas, but also in greens and purples, which are a combination of a blue and another colorant (yellow, red). FORS is not useful in identifying yellow or red organic colorants. | |
==How to look at the results== | ==How to look at the results== | ||
| Line 25: | Line 43: | ||
===Results=== | ===Results=== | ||
| − | Example: | + | '''Example:''' |
| + | {| class="wikitable" style="float:left; margin-right: 30px;" | ||
| + | |- | ||
| + | ! Analysis point !! Image !! Method !! Results | ||
| + | |- | ||
| + | | Pt 1 || [[File:53.21-pt1.png|link=|50px|center]] || XRF || orpiment (EEM to be run) | ||
| + | |- | ||
| + | | Pt 2 || [[File:53.21-pt2.png|link=|50px|center]] || XRF, EEM || inconclusive | ||
| + | |- | ||
| + | | Pt 3 || [[File:53.21-pt3.png|link=|50px|center]] || XRF, EEM || orpiment, turmeric | ||
| + | |- | ||
| + | | Pt 4 || [[File:11.14267-pt2.jpg|link=|50px|center]] || XRF, EEM || safflower (FORS to be run) | ||
| + | |- | ||
| + | | Pt 5 || [[File:11.17878-pt1.jpg|link=|50px|center]] || XRF, FORS || indigo | ||
| + | |} | ||
| + | * Analysis point: Each point corresponds to its circle on the print image, which is where the data was collected using the specified analytical methods. | ||
| + | * Image: This is a 10x image collected by the XRF spectrometer that shows the region of analysis. | ||
| + | * Method: This indicates which analytical methods were used on the region of analysis. | ||
| + | * Results:<br>-The colorants are interpreted from the collected data in conjunction with visual inspection. For example, if arsenic is detected in a yellow area, it is interpreted as orpiment (arsenic trisulfide). | ||
| − | + | :-If more than one colorant is identified, they are listed in alphabetical order. | |
| − | + | :-"Inconclusive" indicates that the data collected was not conclusive enough to identify a colorant. | |
| − | + | :-The additional comment, for example in Pt 1 and Pt 4, indicates that the specified analytical method is scheduled to be conducted in the future. Additionally, Pt 4 is visually a purple color which suggests that it was most likely created from a red and blue colorant but only a red color is listed. This is because FORS which is used to identify blue colorants has not be run; hence the comment, (FORS to be run). If FORS is run in the future and a blue colorant is identified, the result will be updated, but if a blue colorant cannot be identified, the result will only reflect the identified colorant, safflower. For more detail, please see "faded colors" below. | |
| − | |||
| − | |||
===Raw data=== | ===Raw data=== | ||
The below spectra and pattern are examples of the raw data collected by each analytical method. These data are interpreted by conservation scientists experienced in the particular analytical technique to identify the colorant. As such, only the interpreted results, not the raw data, are given for each print. | The below spectra and pattern are examples of the raw data collected by each analytical method. These data are interpreted by conservation scientists experienced in the particular analytical technique to identify the colorant. As such, only the interpreted results, not the raw data, are given for each print. | ||
| + | |||
| + | <gallery mode="packed" heights="200px" style="text-align:left;"> | ||
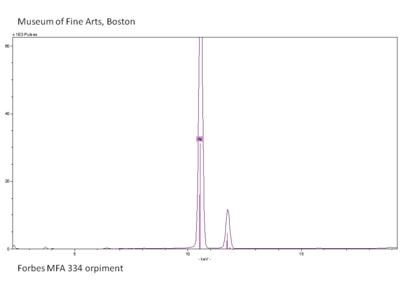
| + | Slide4 FC334.PNG|<center>XRF spectrum of Orpiment</center> | ||
| + | Safflower color.PNG|<center>3D EEM pattern of Safflower</center> | ||
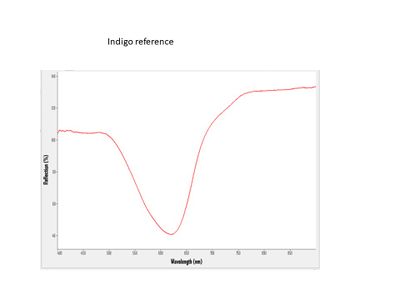
| + | Indigo FORS.JPG|<center>FORS spectrum of Indigo</center> | ||
| + | </gallery> | ||
===What colors cannot be identified?=== | ===What colors cannot be identified?=== | ||
| + | *Faded colors are a challenge and at times not all colors can be fully detected. Although visual observation may give a clue to the colorant or colorants present, only the results from the analytical methods are given. Therefore, if only one colorant of a mixture/overprinting was detected although visually the color is identifiable as a mixture/overprinting, only the detected color is presented in the results. In most cases, XRF, EEM, and FORS still pick up faded colors, but all three techniques are restricted by their detection limits as well as the presence of any fillers and fluorescence in the paper itself. | ||
| + | |||
| + | *[[Flavonoid|Flavonoids]] are also a challenge. This group of organic yellow colorants containing flavonoids cannot be reliably distinguished from one another with the three analytical methods used in this research. While FTIR reflection spectroscopy has been used to differentiate the flavonoids (see [[Bibliography]]) this analytical method was not carried out for this research.<br>Dayflower which can shift to a greenish blue, yellow, or tan, also has a similar EEM fluorescence to the flavonoids once it discolors. Luckily, most times the presence of dayflower can be visually distinguished from the other flavonoids and/or FORS analysis can still detect its presence. | ||
| + | |||
| + | *red lead/lead white | ||
| + | |||
| + | *[[Carbon black]] cannot be detected via the three analytical methods and was not actively pursued during this research. Therefore, carbon black used as a color (black, gray, or blue gray) or mixed with another color is not identified in this database. | ||
| + | |||
| + | *[[Orpiment]] can have different structural forms due to the way it is prepared; directly by grinding the mineral orpiment (natural orpiment) or by heat treating to create a mixture of crystalline and amphorus form (processed orpiment), and synthetic orpiment. The mineral orpiment and synthetic orpiment both have been found in ''ukiyo-e'' prints (see [[Bibliography]]). The identification of these different structural forms of orpiment requires the use of [[Raman spectroscopy]] which was not conducted for this research. | ||
| + | |||
| + | *[[Mica]] While XRF spectroscopy and visual inspection is used to determine the presence of mica, | ||
==Japanese terms for color and materials== | ==Japanese terms for color and materials== | ||
| − | Throughout history, many of the colors and materials have had multiple names and still may have multiple names associated with them. For this database, we have tried to use the most common currently-used name for the raw material used to produce the color. For example, orpiment (石黄 sekiō | + | Throughout history, many of the colors and materials have had multiple names and still may have multiple names associated with them. For this database, we have tried to use the most common currently-used name for the raw material used to produce the color. For example, orpiment (石黄) ''sekiō'' is also commonly called ''kiō''. It has also been called ''yūō'' (雄黄) which is actually the Chinese word for realgar, while the Chinese term for orpiment, 雌黄 (''shiō'' in Japanese) has been also used to describe orpiment as well as gamboge. |
| − | + | [[File:Colorwheel2.png|40px|link=Ukiyo-e Print Colorant Database]] [[Ukiyo-e Print Colorant Database]] | |
Latest revision as of 22:32, 17 August 2024
For this research non-invasive, non-destructive techniques that do not require sampling from the prints were used to analyze the colorants. The analytical techniques used are X-ray Fluorescence (XRF), Excitation Emission Matrix (EEM), and Fiber Optic Reflectance (FORS) spectroscopies. Colorant samples were also formulated and printed using organic and inorganic materials traditionally believed to have been used during the Edo period (1603–1868). These were used to cross reference the analytical results. These methods, in conjunction with visual inspection, were then used to identify the colorants.
Analytical Methods
What is XRF?
X-ray fluorescence (XRF) spectroscopy is a nondestructive analytical method used to qualitatively and semi-quantitatively determine the elemental content of materials. The x-ray spectrum emitted from each element has a unique set of energies that are related to the type and number of atoms present in the sample. For this research, ARTAX open-architecture instrument was used.
XRF is used to identify inorganic colorants based on their inorganic element composition. For example, orpiment can be identified using XRF because it can detect the arsenic in orpiment, which is an arsenic trisulfide. In combination with visual inspection (yellow color) it can be identified as orpiment because it is the only known yellow colorant with arsenic.
Since most XRF cannot detect atomic elements below calcium (i.e. all elements with a smaller atomic number than calcium on the periodic table), it cannot aid in the identification of organic colorants.
What is EEM?
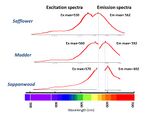
Excitation Emission Matrix (EEM) spectroscopy is a method that results in a 3D pattern of the fluorescence of an organic material. The pattern is produced by plotting the intensity of a sample’s fluorescence emissions occurring as a series of stepped excitation wavelengths. Each material has a reproducible 3D fluorescence pattern that allows its identification. Even the lack of fluorescence, which indicates the material is non-fluorescent, also provides a key piece of information for identification of a colorant. The reference colorant samples were printed to create a set of baseline patterns for each colorant. EEM was used to identify some organic yellow and and most red colorants. For this research, an Agilent Cary Eclipse Spectrometer with a fiber optic probe was used.
|
| Excitation and emission maxima of the three red colorants |

|
| 3D EEM plots of the three red colorants |
Excitation can occur at many wavelengths. When the instrument is set to scan through several excitation wavelengths, it can determine which wavelength excites the most molecules to fluoresce. This is called the Excitation maxima. Exposing the material to this maximum excitation wavelength, the emitted fluorescence or the emission curve can be measured and the point with the highest emission is called the Emission max. The Excitation and Emission maxima of the three reds, safflower, madder, and sappanwood are distinct (see left). Thus the three reds can be distinguished by their fluorescence measurements. The Emission maxima for safflower is in the yellow-orange region, madder is in the orange region, and sappanwood is in the orange-red region.
EEM has been easiest for identifying the reds when the spectral data is shown as a 3-dimensional plot, even in mixtures, where the excitation wavelength is given on the vertical axis and the emission wavelength on the horizontal access. The intensity of the absorption or emission is given by the color with dark red being the most intense. The angled bar on the plot is where the excitation and emission wavelengths are identical, thus the detector is saturated at these points. Shown on the left are the 3D EEM plots for safflower, madder, and sappanwood with their individually distinct patterns.
Additionally some of the yellow in ukiyo-e prints also fluoresce. For example, turmeric has a unique fluorescence or EEM pattern, making it an easily identifiable colorant using EEM. However, some of the other organic yellow colorants such as Japanese pagoda tree (enju) and gardenia (kuchinashi) which belong to a general chemical class known as flavonoids is difficult to reliably differentiate.
What is FORS?
Fiber Optics Reflectance spectroscopy (FORS) is a non-invasive technique that can be used for the examination of color as well as for the identification of colorants. FORS uses a fiber optic probe and a pulsed xenon source to provide good sensitivity, examine small areas, and minimize the interference from ambient light. For this research, an Ocean Optics miniature spectrometer was used.
FORS is able to distinguish between the three common blue colorants used in Japanese woodblock prints: dayflower, indigo, and Prussian blue. This can be accomplished, not only in blue printed areas, but also in greens and purples, which are a combination of a blue and another colorant (yellow, red). FORS is not useful in identifying yellow or red organic colorants.
How to look at the results
The results presented in this database are based on the three analytical methods mentioned above: XRF, FORS, and EEM. XRF was run on all data points, but FORS and EEM were utilized according to the visual color as well as speculated color. For example, FORS was run where blue was speculated (i.e. blues, greens, purples, and faded variations), but would not have been run on yellows or reds. Also, at this time other analytical or photographic methods that can also aid in the identification of colorants have not been used. Therefore, the results are not all-encompassing and/or definitive.
Results
Example:
| Analysis point | Image | Method | Results |
|---|---|---|---|
| Pt 1 |  |
XRF | orpiment (EEM to be run) |
| Pt 2 |  |
XRF, EEM | inconclusive |
| Pt 3 |  |
XRF, EEM | orpiment, turmeric |
| Pt 4 |  |
XRF, EEM | safflower (FORS to be run) |
| Pt 5 |  |
XRF, FORS | indigo |
- Analysis point: Each point corresponds to its circle on the print image, which is where the data was collected using the specified analytical methods.
- Image: This is a 10x image collected by the XRF spectrometer that shows the region of analysis.
- Method: This indicates which analytical methods were used on the region of analysis.
- Results:
-The colorants are interpreted from the collected data in conjunction with visual inspection. For example, if arsenic is detected in a yellow area, it is interpreted as orpiment (arsenic trisulfide).
- -If more than one colorant is identified, they are listed in alphabetical order.
- -"Inconclusive" indicates that the data collected was not conclusive enough to identify a colorant.
- -The additional comment, for example in Pt 1 and Pt 4, indicates that the specified analytical method is scheduled to be conducted in the future. Additionally, Pt 4 is visually a purple color which suggests that it was most likely created from a red and blue colorant but only a red color is listed. This is because FORS which is used to identify blue colorants has not be run; hence the comment, (FORS to be run). If FORS is run in the future and a blue colorant is identified, the result will be updated, but if a blue colorant cannot be identified, the result will only reflect the identified colorant, safflower. For more detail, please see "faded colors" below.
Raw data
The below spectra and pattern are examples of the raw data collected by each analytical method. These data are interpreted by conservation scientists experienced in the particular analytical technique to identify the colorant. As such, only the interpreted results, not the raw data, are given for each print.
What colors cannot be identified?
- Faded colors are a challenge and at times not all colors can be fully detected. Although visual observation may give a clue to the colorant or colorants present, only the results from the analytical methods are given. Therefore, if only one colorant of a mixture/overprinting was detected although visually the color is identifiable as a mixture/overprinting, only the detected color is presented in the results. In most cases, XRF, EEM, and FORS still pick up faded colors, but all three techniques are restricted by their detection limits as well as the presence of any fillers and fluorescence in the paper itself.
- Flavonoids are also a challenge. This group of organic yellow colorants containing flavonoids cannot be reliably distinguished from one another with the three analytical methods used in this research. While FTIR reflection spectroscopy has been used to differentiate the flavonoids (see Bibliography) this analytical method was not carried out for this research.
Dayflower which can shift to a greenish blue, yellow, or tan, also has a similar EEM fluorescence to the flavonoids once it discolors. Luckily, most times the presence of dayflower can be visually distinguished from the other flavonoids and/or FORS analysis can still detect its presence.
- red lead/lead white
- Carbon black cannot be detected via the three analytical methods and was not actively pursued during this research. Therefore, carbon black used as a color (black, gray, or blue gray) or mixed with another color is not identified in this database.
- Orpiment can have different structural forms due to the way it is prepared; directly by grinding the mineral orpiment (natural orpiment) or by heat treating to create a mixture of crystalline and amphorus form (processed orpiment), and synthetic orpiment. The mineral orpiment and synthetic orpiment both have been found in ukiyo-e prints (see Bibliography). The identification of these different structural forms of orpiment requires the use of Raman spectroscopy which was not conducted for this research.
- Mica While XRF spectroscopy and visual inspection is used to determine the presence of mica,
Japanese terms for color and materials
Throughout history, many of the colors and materials have had multiple names and still may have multiple names associated with them. For this database, we have tried to use the most common currently-used name for the raw material used to produce the color. For example, orpiment (石黄) sekiō is also commonly called kiō. It has also been called yūō (雄黄) which is actually the Chinese word for realgar, while the Chinese term for orpiment, 雌黄 (shiō in Japanese) has been also used to describe orpiment as well as gamboge.