Difference between revisions of "Triphenyl phosphate"
| (20 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Description== | ==Description== | ||
| − | An aromatic phosphate that was commonly used as a flame retardant and plasticizer. Triphenyl phosphate was placed on the EPA Toxic Substance list in 2014 as a compound that exhibits acute and chronic aquatic toxicity. As a flame retardant | + | An aromatic phosphate that was commonly used as a [[flame retardant]] and [[plasticizer]]. Triphenyl phosphate (TPhP) was placed on the EPA Toxic Substance list in 2014 as a compound that exhibits acute and chronic aquatic toxicity. As of 2024 its use has not been restricted. Major commercial uses include paints, coatings, adhesives, plastics, rubber products, textiles, and electronics. |
| + | |||
| + | As a flame retardant, TPhP decomposes with heat to form [[phosphoric acid]], then further reacts to form pyrophosphoric acid which acts as a heat transfer blocker. Until the mid-1990s brominated flame retardants (BRFs) were most commonly used. When BFRs were phased out due to environmental concerns, they were replaced by organophosphate flame retardants (OPFR), that are now considered to be the most effective flame retardant. In the production processes, TPhP is physically mixed with materials rather than chemically bonded. Thus, it can migrate out of the material via volatilization, leaching, abrasion or dissolution. | ||
| + | |||
| + | As a non-volatile plasticizer, triphenyl phosphate increases the diffusion coefficients of the solvents thereby decreasing drying times for coatings. | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | TPhP; TPP | |
==Applications== | ==Applications== | ||
| + | * Flame retardant in electronic equipment, building materials, furniture, hydraulic fluids and plastics | ||
* Plasticizer often used in glues, varnishes, nail polishes, and casting resins | * Plasticizer often used in glues, varnishes, nail polishes, and casting resins | ||
| − | |||
| − | |||
| − | |||
| + | ==Risks== | ||
| + | * Low toxicity by dermal and oral contact but some studies have linked TPhP to reproductive and developmental toxicity, neurotoxicity, metabolic disruption, and endocrine effects. | ||
| + | * Considered hazardous waste; Do not dispose via drains. | ||
| + | * Widely detected in sediment, soil, indoor dust, and air due to its extensive use. | ||
| + | * Biodegrades in water but is considered toxic to aquatic organisms potentially producing long-term adverse effects. | ||
| + | * ThermoFisher: [https://assets.thermofisher.com/DirectWebViewer/private/document.aspx?prd=ACR14767~~PDF~~MTR~~KOSD~~EN~~2022-05-27%2018:34:45~~Triphenyl%20phosphate~~ SDS] | ||
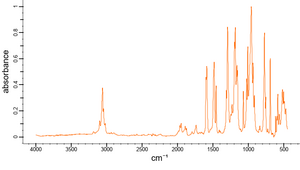
| + | [[[SliderGallery rightalign|Wiley_TPP_FTIR.png~FTIR (Wiley)]]] | ||
==Physical and Chemical Properties== | ==Physical and Chemical Properties== | ||
* Chemical formula = C18H15O4P | * Chemical formula = C18H15O4P | ||
* Molecular weight = 326.288 g·mol−1 | * Molecular weight = 326.288 g·mol−1 | ||
* Appearance = Colorless solid | * Appearance = Colorless solid | ||
| − | * Density = 1. | + | * Density = 1.2-1.3 g/mL |
| − | * Melting point = | + | * Melting point = 47-53 °C (116-127 °F) |
* Boiling point = 244 °C (471 °F) | * Boiling point = 244 °C (471 °F) | ||
| + | * Water solubility = 0.7-1.9 mg/L | ||
| + | * Refractive Index = 1.550 | ||
==Resources and Citations== | ==Resources and Citations== | ||
* Wikipedia: [https://en.wikipedia.org/wiki/Triphenyl_phosphate Triphenyl phosphate] Accessed Mar 2024 | * Wikipedia: [https://en.wikipedia.org/wiki/Triphenyl_phosphate Triphenyl phosphate] Accessed Mar 2024 | ||
| − | + | * European Chemicals Agency (ECHA): [https://echa.europa.eu/documents/10162/d6e2e15e-41af-b344-90c3-3c8875aa4b31 Identification of Triphenyl Phosphate] | |
| + | * Environmental Protection Agency (EPA): [https://www.epa.gov/sites/default/files/2020-09/documents/casrn_115-86-6_triphenyl_phosphate_tpp_final_scope.pdf Risk Evaluation for Triphenyl Phosphate] | ||
| + | * Centers for Disease Control and Prevention: [https://www.cdc.gov/niosh/idlh/115866.html Triphenyl phosphate] | ||
| + | * Zhongshi Hong, Yachen Li, Xian Deng, Mingliang Chen, Jianpeng Pan, Zhichuan Chen, Xu Zhang, Chunxiao Wang, Chengzhi Qiu, 'Comprehensive analysis of triphenyl phosphate: An environmental explanation of colorectal cancer progression', ''Ecotoxicology and Environmental Safety'', Vol. 241, 2022, p.113778. [https://www.sciencedirect.com/science/article/pii/S0147651322006182 Link] | ||
| + | * Raj Kumar Arya, Harmandeep Kaur, Manju Rawat, Jyoti Sharma, Avinash Chandra, Sanjeev Ahuja, 'Influence of plasticizer (triphenyl phosphate) loading on drying of binary coatings: Poly(styrene)-p-xylene coatings', ''Progress in Organic Coatings'', Vol. 150, 2021, p.106001. [https://www.sciencedirect.com/science/article/pii/S0300944020312121 Link] | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:32, 24 March 2024
Description
An aromatic phosphate that was commonly used as a Flame retardant and Plasticizer. Triphenyl phosphate (TPhP) was placed on the EPA Toxic Substance list in 2014 as a compound that exhibits acute and chronic aquatic toxicity. As of 2024 its use has not been restricted. Major commercial uses include paints, coatings, adhesives, plastics, rubber products, textiles, and electronics.
As a flame retardant, TPhP decomposes with heat to form Phosphoric acid, then further reacts to form pyrophosphoric acid which acts as a heat transfer blocker. Until the mid-1990s brominated flame retardants (BRFs) were most commonly used. When BFRs were phased out due to environmental concerns, they were replaced by organophosphate flame retardants (OPFR), that are now considered to be the most effective flame retardant. In the production processes, TPhP is physically mixed with materials rather than chemically bonded. Thus, it can migrate out of the material via volatilization, leaching, abrasion or dissolution.
As a non-volatile plasticizer, triphenyl phosphate increases the diffusion coefficients of the solvents thereby decreasing drying times for coatings.
Synonyms and Related Terms
TPhP; TPP
Applications
- Flame retardant in electronic equipment, building materials, furniture, hydraulic fluids and plastics
- Plasticizer often used in glues, varnishes, nail polishes, and casting resins
Risks
- Low toxicity by dermal and oral contact but some studies have linked TPhP to reproductive and developmental toxicity, neurotoxicity, metabolic disruption, and endocrine effects.
- Considered hazardous waste; Do not dispose via drains.
- Widely detected in sediment, soil, indoor dust, and air due to its extensive use.
- Biodegrades in water but is considered toxic to aquatic organisms potentially producing long-term adverse effects.
- ThermoFisher: SDS
Physical and Chemical Properties
- Chemical formula = C18H15O4P
- Molecular weight = 326.288 g·mol−1
- Appearance = Colorless solid
- Density = 1.2-1.3 g/mL
- Melting point = 47-53 °C (116-127 °F)
- Boiling point = 244 °C (471 °F)
- Water solubility = 0.7-1.9 mg/L
- Refractive Index = 1.550
Resources and Citations
- Wikipedia: Triphenyl phosphate Accessed Mar 2024
- European Chemicals Agency (ECHA): Identification of Triphenyl Phosphate
- Environmental Protection Agency (EPA): Risk Evaluation for Triphenyl Phosphate
- Centers for Disease Control and Prevention: Triphenyl phosphate
- Zhongshi Hong, Yachen Li, Xian Deng, Mingliang Chen, Jianpeng Pan, Zhichuan Chen, Xu Zhang, Chunxiao Wang, Chengzhi Qiu, 'Comprehensive analysis of triphenyl phosphate: An environmental explanation of colorectal cancer progression', Ecotoxicology and Environmental Safety, Vol. 241, 2022, p.113778. Link
- Raj Kumar Arya, Harmandeep Kaur, Manju Rawat, Jyoti Sharma, Avinash Chandra, Sanjeev Ahuja, 'Influence of plasticizer (triphenyl phosphate) loading on drying of binary coatings: Poly(styrene)-p-xylene coatings', Progress in Organic Coatings, Vol. 150, 2021, p.106001. Link