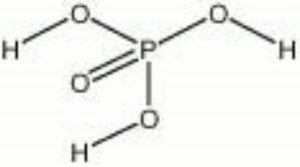
Phosphoric acid
Jump to navigation
Jump to search
Description
A colorless, odorless crystalline solid that is usually sold as an aqueous solution. Phosphoric acid is produced from phosphate rock deposits by either a wet process or a heat process. It is used in the manufacture of Sugar, Gelatin, soaps, detergents, polishes, fertilizers, and pharmaceuticals. Phosphoric acid is also used to an etch stone or metal lithograph plates. Phosphoric acid solutions (10-20%) have also been used to clean Iron, Copper, Brass, and Bronze (Stambolov et. al 1987).
Synonyms and Related Terms
orthophosphoric acid; phosphorous dephlogisticated
Physical and Chemical Properties
Soluble in water, ethanol.
pH of 0.1N solution=1.5
| Composition | H3PO4 |
|---|---|
| CAS | 7664-38-2 |
| Melting Point | 42.35 C |
| Density | 1.834 g/ml |
| Molecular Weight | mol. wt. = 98.0 |
Hazards and Safety
Toxic by ingestion and inhalation. Burns skin and eyes on contact. Corrosive to iron containing metals.
Resources and Citations
- Stambolov, Bleck, Eichelmann, Korrosion ud Konservierung von Kunst ind Kulturgut aus Metall, Weimar, 1987.(Corrosion and Conservation of Metallic Artworks and Culture Material)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 602
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7500
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH of 0.1N solution=1.5
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Goran Budja, Contributed information, Feb. 2006.