Difference between revisions of "Glutaraldehyde"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|glutaraldehyde.jpg~Chemical structure]]] | [[[SliderGallery rightalign|glutaraldehyde.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Toxic by inhalation and ingestion. Skin contact causes irritation. | ||
| + | |||
| + | Fisher Scientific: [https://fscimage.fishersci.com/msds/10421.htm MSDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water. | Soluble in water. | ||
| Line 37: | Line 42: | ||
|} | |} | ||
| − | == | + | == Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 49: | Line 48: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4480 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4480 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Glutaraldehyde (Accessed Mar. 1, 2006) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 09:39, 19 August 2020
Description
A strong smelling gas sold commercially as a 25% aqueous solution. Glutaraldehyde polymerizes in water to form a clear viscous liquid. It is used as a biocide which acts by disrupting the cells of Lichen, mold, bacteria, and fungi by alkylating and crosslinking the proteins. Glutaraldehyde is also used as a synthetic tanning agent for leathers.
Synonyms and Related Terms
glutaric dialdehyde; 1,5-pentanedial; glutaral; 1,3-diformylpropane; Cidex; Glutarol; Novaruca; Verucasep; Ucaricide
Risks
Toxic by inhalation and ingestion. Skin contact causes irritation.
Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water.
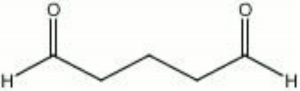
| Composition | OHC(CH2)3CHO |
|---|---|
| CAS | 111-30-8 |
| Melting Point | -14 |
| Density | 0.7 |
| Molecular Weight | mol. wt. = 100.12 |
| Refractive Index | 1.4338 |
| Boiling Point | 187-189 (dec) |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4480
- Wikipedia: http://en.wikipedia.org/wiki/Glutaraldehyde (Accessed Mar. 1, 2006)