Difference between revisions of "Whewellite"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 9: | Line 9: | ||
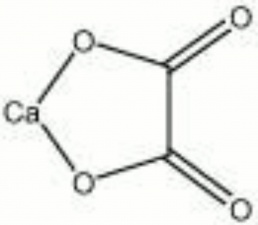
[[[SliderGallery rightalign|whewellite.jpg~Chemical structure]]] | [[[SliderGallery rightalign|whewellite.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/80705.htm MSDS] | |
| − | + | ==Physical and Chemical Properties== | |
| − | Luster=vitreous to pearly. Streak = white. Fluorescent in UV light. | + | * Soluble in dilute mineral acids. Insoluble in water, acetic acid. |
| + | * Slightly hygroscopic. | ||
| + | * Transparent to translucent tabular crystals with good cleavage in all three planes. | ||
| + | * Luster=vitreous to pearly. | ||
| + | * Streak = white. | ||
| + | * Fluorescent in UV light. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 29: | Line 34: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.2 | + | | 2.2 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 35: | Line 40: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Whewellite.shtml Whewellite] | |
| − | + | * M. del Monte, C. Sabbioni, G.Zappia. The origin of calcium oxalates on historical buildings, monuments and natural outcrops. The science of the total environment 67, (1987), pp. 17-39 | |
| − | + | * B. Ford, I.MacLeod, P.Haydock, "Rock art pigments from Kimberley region of Western Australia: identification of the minerals and conversion mechanisms." ''Studies in conservation'' 39, no. 1 (1994), pp. 57-69 | |
| − | |||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | + | * Wikipedia,: http://en.wikipedia.org/wiki/Whewellite (Accessed Nov. 29, 2005) | |
| − | |||
| − | * Wikipedia, | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 14:56, 26 June 2022
Description
A white to colorless mineral composed of Calcium oxalate monohydrate. Whewellite is formed on the surface of Marble and Limestone due to the presence of microorganisms, such as Lichen.
Synonyms and Related Terms
calcium oxalate monohydrate; calcium salt of ethanedioic acid; wewelita (Esp.); whewelite (Port.); Whewellit (Deut.)
Risks
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Soluble in dilute mineral acids. Insoluble in water, acetic acid.
- Slightly hygroscopic.
- Transparent to translucent tabular crystals with good cleavage in all three planes.
- Luster=vitreous to pearly.
- Streak = white.
- Fluorescent in UV light.
| Composition | Ca(C2O4)-H2O |
|---|---|
| CAS | 5794-28-5 |
| Mohs Hardness | 2.5-3.0 |
| Density | 2.2 g/ml |
| Molecular Weight | mol. wt. = 146.11 |
Resources and Citations
- Mineralogy Database: Whewellite
- M. del Monte, C. Sabbioni, G.Zappia. The origin of calcium oxalates on historical buildings, monuments and natural outcrops. The science of the total environment 67, (1987), pp. 17-39
- B. Ford, I.MacLeod, P.Haydock, "Rock art pigments from Kimberley region of Western Australia: identification of the minerals and conversion mechanisms." Studies in conservation 39, no. 1 (1994), pp. 57-69
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Wikipedia,: http://en.wikipedia.org/wiki/Whewellite (Accessed Nov. 29, 2005)