Difference between revisions of "Ferric sulfate"
Jump to navigation
Jump to search
| Line 5: | Line 5: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate | + | iron III sulfate; ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate |
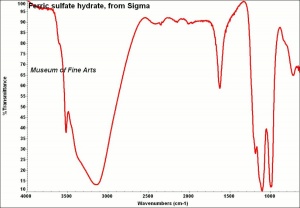
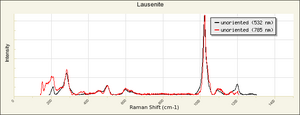
[[[SliderGallery rightalign|FerricsulfateSigmaIR.jpg~FTIR|Lausenite Raman RRUFF X070004.png~Raman (U of Arizona)]]] | [[[SliderGallery rightalign|FerricsulfateSigmaIR.jpg~FTIR|Lausenite Raman RRUFF X070004.png~Raman (U of Arizona)]]] | ||
Revision as of 10:51, 9 December 2022
Description
Grayish-white powder formed by adding Sulfuric acid to Ferric hydroxide. Ferric sulfate is very Hygroscopic. It is used as a mordant in textile dyeing and as a component in iron gall inks. Ferric sulfate is also used in water purification systems. Ferric sulfate occurs naturally in minerals with varying states of hydration, including lausenite [Fe2(SO4)3-5H2O], Kornelite [Fe2(SO4)3-7H2O], Coquimbite [Fe2(SO4)3-9H2O], and Quenstedtite [Fe2(SO4)3-10H2O].
Synonyms and Related Terms
iron III sulfate; ferric sulphate (Br.); ferric persulfate; ferric sesquisulfate; ferric tersulfate
Risks
- Non-combustible.
- Decomposes with light
- Fisher Scientific: SDS
Physical and Chemical Properties=
Slightly soluble in water and alcohol. Insoluble in organic solvents.
| Composition | Fe2(SO4)3 |
|---|---|
| CAS | 10028-22-5 |
| Melting Point | 480 C (d) |
| Density | 2.0-2.1 g/ml |
| Molecular Weight | 399.88 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3963