Difference between revisions of "Titanium white"
(username removed) |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A durable white paint pigment used in the 20th century. Titanium white is composed of [ | + | A durable white paint pigment used in the 20th century. Titanium white is composed of [[titanium dioxide]]. It is a very stable compound with a high refractive index. Titanium white pigment was prepared from ground [[rutile]] as early as 1870. Synthetic titanium dioxide was first prepared in 1906. In 1916, it was manufactured simultaneously in Norway (Titan Co.) and the United States (Titanium Pigment Corp.). The pigment was sold under the name of Titanox and contained 25% titanium dioxide co-precipitated with 75% [[calcium sulfate]], or [[barium sulfate]]. Both pure titanium and barium base titanium pigments have a very fine texture. Prior to 1938, only [[anatase]] was synthetically produced. However, white paints with anatase were subject to chalking and yellowing, so manufacturing shifted to the use of rutile in 1938 and only rutile white pigments were commercially produced since 1940. Currently, titanium pigments are usually sold as admixtures with zinc oxide. In addition to paints, titanium dioxide is used as a pigment in paper coatings, textiles, leather, ink, glass, and ceramic glazes. |
[[File:titanum_white_Vegvari.jpg|thumb|Titanium white pigments]] | [[File:titanum_white_Vegvari.jpg|thumb|Titanium white pigments]] | ||
| + | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 13:14, 31 July 2014
Description
A durable white paint pigment used in the 20th century. Titanium white is composed of Titanium dioxide. It is a very stable compound with a high refractive index. Titanium white pigment was prepared from ground Rutile as early as 1870. Synthetic titanium dioxide was first prepared in 1906. In 1916, it was manufactured simultaneously in Norway (Titan Co.) and the United States (Titanium Pigment Corp.). The pigment was sold under the name of Titanox and contained 25% titanium dioxide co-precipitated with 75% Calcium sulfate, or Barium sulfate. Both pure titanium and barium base titanium pigments have a very fine texture. Prior to 1938, only Anatase was synthetically produced. However, white paints with anatase were subject to chalking and yellowing, so manufacturing shifted to the use of rutile in 1938 and only rutile white pigments were commercially produced since 1940. Currently, titanium pigments are usually sold as admixtures with zinc oxide. In addition to paints, titanium dioxide is used as a pigment in paper coatings, textiles, leather, ink, glass, and ceramic glazes.
Synonyms and Related Terms
Titanox; titanium dioxide; titania; Pigment White 6; CI 77891; dioxyde de titane (Fr.); blanc de titane (Fr.); Titandioxid (Deut., Sven.); bianco di titanio (It.); dióxido de titanio (Esp.); titandioksid (Nor.);titaanwit (Ned.); Titanweiss (Deut.)
Other Properties
Particle size = 0.2-0.5 micrometers.
Fluoresces a deep purple in ultraviolet light ; mixtures with zinc oxide fluoresce green-yellow.
Resistant to acids and alkalis. Insoluble in water.
| Composition | TiO2 |
|---|---|
| CAS | 13463-67-7 |
| Melting Point | 1640 (dec) |
| Density | 4.26 |
| Refractive Index | 2.5-2.7 |
Hazards and Safety
Nontoxic. Noncombustible.
No significant hazards.
LINK: International Chemical Safety Card
Additional Information
° M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
Comparisons
Characteristics of Common White Pigments
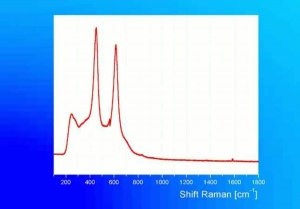
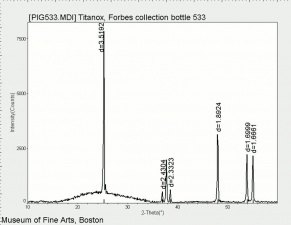
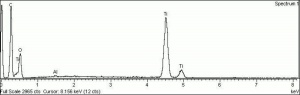
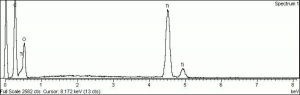
Additional Images
Authority
- Website address 1 Comment: Pigments Through the Ages -http://webexhibits.org/pigments/indiv/overview/tiwhite.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000