Difference between revisions of "Sebacic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White flaky crystals. Sebacic acid is obtained from heating [ | + | White flaky crystals. Sebacic acid is obtained from heating [[castor%20oil|castor oil]] with [[sodium%20hydroxide|sodium hydroxide]]. It is used in the synthesis of [[alkyd%20resin|alkyd]], [[nylon%20resin|nylon]], and [[polyester%20resin|polyester]] resins. Sebacic acid is also used as a [[plasticizer|plasticizer]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 11:08, 10 May 2016
Description
White flaky crystals. Sebacic acid is obtained from heating Castor oil with Sodium hydroxide. It is used in the synthesis of alkyd, nylon, and polyester resins. Sebacic acid is also used as a Plasticizer.
Synonyms and Related Terms
1,8-octanedicarboxylic acid; sebacylic acid; decanedioic acid
Other Properties
Soluble in ethanol, ether. Slightly soluble in water.
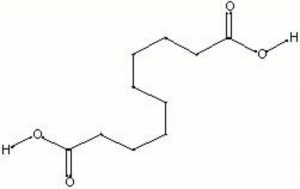
| Composition | HOOC(CH2)8COOH |
|---|---|
| CAS | 111-20-6 |
| Melting Point | 133-134.5 |
| Density | 1.110-1.207 |
| Molecular Weight | mol. wt.=102.07 |
| Refractive Index | 1.422 |
| Boiling Point | 295.0 |
Hazards and Safety
Combustible. Inhalation, ingestion and skin contact may cause irritation.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8558