Difference between revisions of "Vermiculite"
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in [[plaster|plaster]], [[concrete|concrete]], [[brick|brick]], [[rubber | + | A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in [[plaster|plaster]], [[concrete|concrete]], [[brick|brick]], [[rubber|rubber]], [[soil|soil]], [[paper|paper]], [[paint|paint]], and [[plastic|plastics]]. |
[[File:vermiculite2.jpg|thumb|Vermiculite, expanded]] | [[File:vermiculite2.jpg|thumb|Vermiculite, expanded]] | ||
| Line 10: | Line 10: | ||
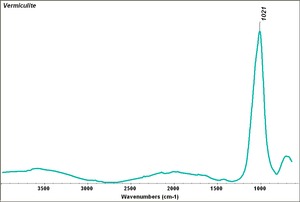
[[[SliderGallery rightalign|Vermiculite.TIF~FTIR (MFA)]]] | [[[SliderGallery rightalign|Vermiculite.TIF~FTIR (MFA)]]] | ||
| + | |||
| + | == Risks == | ||
| + | |||
| + | Vermiculite mined prior to 1990 may contain asbestos which is toxic by ingestion and inhalation. | ||
| + | |||
| + | Noncombustible. Resistant to insects, bacteria, and fungi. | ||
| + | |||
| + | Milllipore Sigma: [https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=Z765422&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fz765422%3Flang%3Den SDS] | ||
== Other Properties == | == Other Properties == | ||
| Line 31: | Line 39: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Vermiculite." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Vermiculite." (Accessed 16 Mar. 2004). |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Vermiculite (Accessed Sept. 20, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 57: | Line 57: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London | + | * Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London. |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 13:48, 5 August 2020
Description
A laminar micaceous mineral composed of hydrated magnesium aluminum iron silicate. Vermiculite occurs naturally as a compact ore. It is mined in Russia, Australia, Brazil, South Africa, and the U.S. (Montana, North Carolina, South Carolina, Wyoming, Colorado). When vermiculite is heated to about 300 C (570 F), it expands to form highly porous, worm-shaped curls of connected mica-like plates. Expanded vermiculite is used as a fire-resistant insulator, spill absorbent, and packing material. It is also used as a lightweight filler in Plaster, Concrete, Brick, Rubber, Soil, Paper, Paint, and plastics.
Synonyms and Related Terms
hydrated magnesium aluminum iron silicate; exfoliated hydrobiotite; Zonolite insulation; Microfil; Microlite; Verxite
Risks
Vermiculite mined prior to 1990 may contain asbestos which is toxic by ingestion and inhalation.
Noncombustible. Resistant to insects, bacteria, and fungi.
Milllipore Sigma: SDS
Other Properties
Unaffected by water, acids, alkalis or organic solvents.
Can expand 6-20 times when heated. Expanded vermiculite can absorb 200-500% of its weight in liquid.
Fracture = unvevn Crystal system = monoclinic Cleavage = perfect Streak = pale yellow
| CAS | 1318-00-9 |
|---|---|
| Mohs Hardness | 2-3 |
| Density | 0.04-0.15 (expanded) |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 10095
- Encyclopedia Britannica, http://www.britannica.com Comment: Vermiculite." (Accessed 16 Mar. 2004).
- Wikipedia: http://en.wikipedia.org/wiki/Vermiculite (Accessed Sept. 20, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Ceramics and Glass Conservation Section, List of Workshop Materials, The British Museum, London.