Difference between revisions of "Zinc white"
| Line 21: | Line 21: | ||
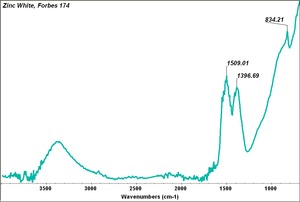
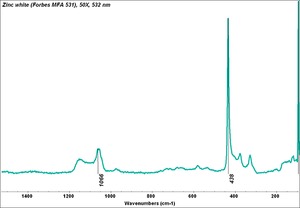
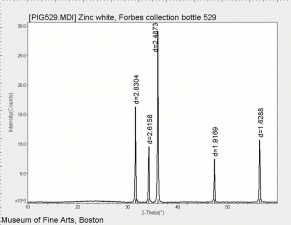
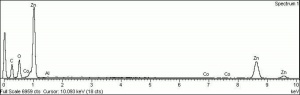
[[[SliderGallery rightalign|Zinc White, Forbes 174.TIF~FTIR (MFA)|Zinc white (Forbes MFA 531), 50X, 532 nm copy.tif~Raman (MFA)|PIG529.jpg~XRD (MFA)|f529sem.jpg~SEM (MFA)|f529edsbw.jpg~EDS(MFA)]]] | [[[SliderGallery rightalign|Zinc White, Forbes 174.TIF~FTIR (MFA)|Zinc white (Forbes MFA 531), 50X, 532 nm copy.tif~Raman (MFA)|PIG529.jpg~XRD (MFA)|f529sem.jpg~SEM (MFA)|f529edsbw.jpg~EDS(MFA)]]] | ||
| − | + | == Risks == | |
Noncombustible. Nonpoisonous, but slightly antiseptic. Inhalation or ingestion of dust may cause slight irritation. Zinc oxide fumes from firing may cause metal fume fever. Reacts violently with aluminum and magnesium powders. | Noncombustible. Nonpoisonous, but slightly antiseptic. Inhalation or ingestion of dust may cause slight irritation. Zinc oxide fumes from firing may cause metal fume fever. Reacts violently with aluminum and magnesium powders. | ||
| Line 28: | Line 28: | ||
U.S Zinc: [http://www.uszinc.com/assets/uploads/2017/08/US-Zinc-SDS-ZnO-Rev10.pdf SDS] | U.S Zinc: [http://www.uszinc.com/assets/uploads/2017/08/US-Zinc-SDS-ZnO-Rev10.pdf SDS] | ||
| + | |||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
Revision as of 14:56, 27 October 2020
Description
A bright, white pigment composed of Zinc oxide. Zinc white is permanent, opaque, and nontoxic. It was known as a white compound since the Middle Ages but was rarely used as a pigment until 1834 when zinc oxide was first listed by Winsor and Newton as a watercolor pigment called Chinese white. It quickly became a standard in watercolor paints but the early batches of zinc white were inferior to Lead white in oil paints because of poor covering and drying properties. However, by the last quarter of the 19th century zinc white was sufficiently improved and became a widely used alternative to lead white in oil paints (Mayer 1969). The following grades of zinc white were sold:
- white seal: purest (>99% zinc oxide) but poor covering
- green seal: greater than 99% zinc oxide with better covering power
- red seal: slightly less pure, generally used for grounds
- gold seal: similar to red seal
- gray seal: contains some metallic zinc.
Zinc oxide is used as a pigment in oil, and in watercolor paints, ceramic glazes, printing inks, glass colorant, UV absorber, Fungicide.
See also French process zinc oxide.
Synonyms and Related Terms
zinc oxide; Pigment White 4; CI 77947; Zinkweiss (Deut.); Schneeweiss (Deut.); Pergamentweiss (Deut.); blanc de zinc (Fr.); bianco di zinco (It.); blanco de cinc (Esp.); zincwitt (Ned.); sinkkivalkoinen (Fin.); branco de zinco (Port.); Chinese white; French zinc; absolute white; snow white; philosophers' wool; nil alba; nibil album; nix album; flowers of zinc; snow flowers; Florence zinc oxide; constant white; Hubbocks white
Risks
Noncombustible. Nonpoisonous, but slightly antiseptic. Inhalation or ingestion of dust may cause slight irritation. Zinc oxide fumes from firing may cause metal fume fever. Reacts violently with aluminum and magnesium powders.
Oil paints with zinc oxide may yellow and chalk with UV exposure.
U.S Zinc: SDS
Physical and Chemical Properties
Soluble in acids and alkalis. Insoluble in water and ethanol.
Autofluoresces yellow.
| Composition | ZnO |
|---|---|
| CAS | 1314-13-2 |
| Melting Point | 1975 |
| Density | 5.47-5.65 |
| Molecular Weight | mol. wt. = 81.4 |
| Refractive Index | 2.02; 2.00 |
| Boiling Point | 7 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Artists' Pigments: A Handbook of their History and Characteristics, R.L.Feller, ed., Cambridge University Press, London, Vol. 1, 1986 Comment: H. Kuhn, "Zinc White"
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Website address 1 Comment: Pigments Through the Ages. - http://webexhibits.org/pigments/indiv/technical/zincwhite.html
- Website address 2 Comment: http://www.coloria.net/varita.htm - 1746
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 887
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Zinc_white (Accessed Nov. 29, 2005)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000