Difference between revisions of "Ethyl acetate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A colorless, liquid with a pleasant fruity smell. Ethyl acetate is [[hygroscopic]] and slowly decomposes in the presence of water to form acids. Ethyl acetate is primarily used as a [[solvent]] for [[cellulose nitrate]], paints, varnishes, lacquers, and airplane dopes. It is also used in the manufacture of photographic films, rayon, synthetic leather, perfumes, and flavorings. | A colorless, liquid with a pleasant fruity smell. Ethyl acetate is [[hygroscopic]] and slowly decomposes in the presence of water to form acids. Ethyl acetate is primarily used as a [[solvent]] for [[cellulose nitrate]], paints, varnishes, lacquers, and airplane dopes. It is also used in the manufacture of photographic films, rayon, synthetic leather, perfumes, and flavorings. | ||
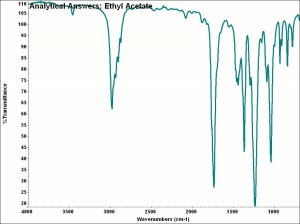
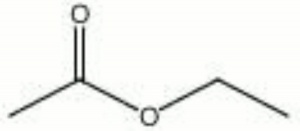
| − | + | [[[SliderGallery rightalign|AAIethylacetate.jpg~FTIR|ethyl acetate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
ethyl ethanoate; acetic ether; acetic ester; vinegar naphtha | ethyl ethanoate; acetic ether; acetic ester; vinegar naphtha | ||
| − | + | == Risks == | |
| − | == | + | * Flammable. |
| + | * Toxic by inhalation and skin contact. | ||
| + | * Irritating to skin, nose and eyes. | ||
| + | * Produces hazy films in humid environments. | ||
| + | * Cisco Chem: [http://www.ciscochem.com/assets/ethyl-acetate-sds.pdf SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Miscible in ethanol, acetone, chloroform, ether. Slightly soluble in water. | Miscible in ethanol, acetone, chloroform, ether. Slightly soluble in water. | ||
| Line 24: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -83 | + | | -83 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.898 | + | | 0.898 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 41: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 77 | + | | 77 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 51: | Line 48: | ||
[[media:download_file_127.pdf|Properties of Common Solvents]] | [[media:download_file_127.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 14:57, 5 August 2022
Description
A colorless, liquid with a pleasant fruity smell. Ethyl acetate is Hygroscopic and slowly decomposes in the presence of water to form acids. Ethyl acetate is primarily used as a Solvent for Cellulose nitrate, paints, varnishes, lacquers, and airplane dopes. It is also used in the manufacture of photographic films, rayon, synthetic leather, perfumes, and flavorings.
Synonyms and Related Terms
ethyl ethanoate; acetic ether; acetic ester; vinegar naphtha
Risks
- Flammable.
- Toxic by inhalation and skin contact.
- Irritating to skin, nose and eyes.
- Produces hazy films in humid environments.
- Cisco Chem: SDS
Physical and Chemical Properties
Miscible in ethanol, acetone, chloroform, ether. Slightly soluble in water.
Hygroscopic. Reacts with water to form acetic acid and alcohol.
| Composition | CH3COOC2H5 |
|---|---|
| CAS | 141-78-6 |
| Melting Point | -83 C |
| Density | 0.898 g/ml |
| Molecular Weight | mol. wt. = 88.1 |
| Refractive Index | 1.370 |
| Boiling Point | 77 C |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3803
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.370