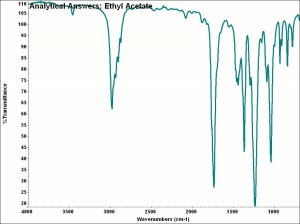
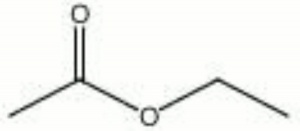
Ethyl acetate
Jump to navigation
Jump to search
Description
A colorless, liquid with a pleasant fruity smell. Ethyl acetate is Hygroscopic and slowly decomposes in the presence of water to form acids. Ethyl acetate is primarily used as a Solvent for Cellulose nitrate, paints, varnishes, lacquers, and airplane dopes. It is also used in the manufacture of photographic films, rayon, synthetic leather, perfumes, and flavorings.
Synonyms and Related Terms
ethyl ethanoate; acetic ether; acetic ester; vinegar naphtha
Risks
- Flammable.
- Toxic by inhalation and skin contact.
- Irritating to skin, nose and eyes.
- Produces hazy films in humid environments.
- Cisco Chem: SDS
- EPA lists ethyl acetate as hazardous waste due to ignitability; concentrations over 10% must be disposed of appropriately
- Other sources list ethyl acetate as a green solvent because it biodegrades quickly and has low toxicity to rodents and fish
Physical and Chemical Properties
Miscible in ethanol, acetone, chloroform, ether. Slightly soluble in water.
Hygroscopic. Reacts with water to form acetic acid and alcohol.
| Composition | CH3COOC2H5 |
|---|---|
| CAS | 141-78-6 |
| Melting Point | -83 C |
| Density | 0.898 g/ml |
| Molecular Weight | mol. wt. = 88.1 |
| Refractive Index | 1.370 |
| Boiling Point | 77 C |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3803
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.370