Difference between revisions of "Methyl ethyl ketone"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to " $2") |
|||
| Line 16: | Line 16: | ||
2-butanone; MEK; ethyl methyl ketone; ethylmethyl ketone; 2-oxobutane; methyl acetone; methylethyl ketone | 2-butanone; MEK; ethyl methyl ketone; ethylmethyl ketone; 2-oxobutane; methyl acetone; methylethyl ketone | ||
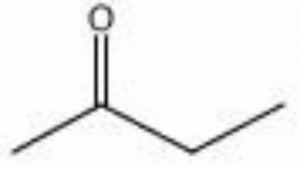
| + | [[[SliderGallery rightalign|methyl ethyl ketone.jpg~Chemical structure]]] | ||
| + | |||
| + | ==Risks == | ||
| − | [ | + | * Flammable. Flash point = -9C Dangerous fire risk. Explosive in limits air 2-10%. |
| + | * Toxic by inhalation. | ||
| + | * Skin contact causes dermatitis. | ||
| + | * Ingestion causes vomiting and abdominal pain. | ||
| + | * Flinn Scientific: [https://www.flinnsci.com/sds_511-methyl-ethyl-ketone/sds_511/ SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Miscible with water, ethanol, ether, benzene and oils. | Miscible with water, ethanol, ether, benzene and oils. | ||
| Line 32: | Line 39: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -86.4 | + | | -86.4 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.805 | + | | 0.805 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 44: | Line 51: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 79.6 | + | | 79.6 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 57: | Line 58: | ||
[[media:download_file_135.pdf|Properties of Common Solvents]] | [[media:download_file_135.pdf|Properties of Common Solvents]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: acts well on cellulose acetate | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: acts well on cellulose acetate | ||
Revision as of 14:43, 18 October 2022
Description
A colorless liquid Solvent with an acetone-like odor. Methyl ethyl ketone, or MEK, is used as a solvent for Cellulose nitrate lacquers, vinyl films, acrylic coatings, inks, and alkyd ( Glyptal) resins. It is also used as a component in paint removers and dry cleaning fluids. MEK does not dissolve Cellulose acetate or most waxes.
Synonyms and Related Terms
2-butanone; MEK; ethyl methyl ketone; ethylmethyl ketone; 2-oxobutane; methyl acetone; methylethyl ketone
Risks
- Flammable. Flash point = -9C Dangerous fire risk. Explosive in limits air 2-10%.
- Toxic by inhalation.
- Skin contact causes dermatitis.
- Ingestion causes vomiting and abdominal pain.
- Flinn Scientific: SDS
Physical and Chemical Properties
Miscible with water, ethanol, ether, benzene and oils.
| Composition | CH3COCH2CH3 |
|---|---|
| CAS | 78-93-3 |
| Melting Point | -86.4 C |
| Density | 0.805 g/ml |
| Molecular Weight | mol. wt.= 72.12 |
| Refractive Index | 1.379 |
| Boiling Point | 79.6 C |
Comparisons
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: acts well on cellulose acetate
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: does not dissolve cellulose acetate and most waxes
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6149; ref. index=1.379
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.377