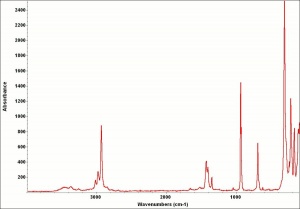
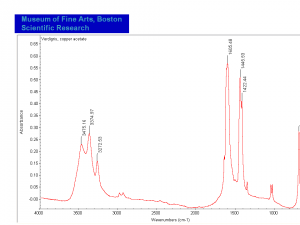
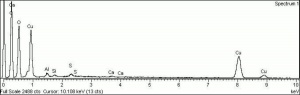
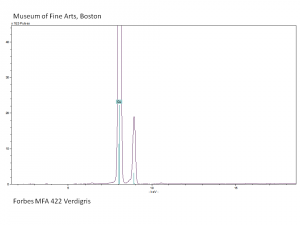
Verdigris
Description
A dark bluish-green pigment composed of basic copper acetate. Verdigris has been manufactured since ancient times by placing copper plates over vats of fermenting grape skins. The acetic acid quickly reacts to form basic copper acetate. When used directly as a pigment, verdigris can discolor from green to black in oil paints, fade in watercolor paints, and react with a paper support. Thus, it is more often used to make copper resinate and as a drier for linseed oil. Verdigris has also been used to dye fabrics and is still used as a colorant and fungicide in antifouling paints.
Historically, the various types of copper corrosion products were not differentiated but rather lumped together and called Aerugo or verdigris. In the 20th century, this practice has carried over with the use of verdigris as a common, though chemically incorrect, name for natural green patinas formed on outdoor Copper, Brass, and Bronze. Depending on atmospheric pollutants, however, these corrosion products are typically composed of Copper sulfate, Copper chloride, or Basic copper carbonate.
Synonyms and Related Terms
basic copper acetate; Pigment Green 20; vert-de-gris (Fr.); Verdigris (Deut.); Grünspan (Deut.); verdigris (It., Ned., Port.); verderame (It.); cardenillo (Esp.), verdete (Esp.); aerugo (Lat.); viride aeris (Lat.); aeruca; zangar; Spanish green; Van Eyck green; copper green; copper rust; copper subacetate; Montpelier green
Other Properties
Pleochroic changing from pale green to dark blue. Strongly birefringent.
Tabular crystals with rhombic and hexagonal faces.
Soluble in acids. slightly soluble in water. Decomposes with heat to produce acetic acid fumes and black residue.
| Composition | Cu(C2H3O2)2-2Cu(OH)2 |
|---|---|
| Refractive Index | 1.53; 1.56 |
Hazards and Safety
Toxic by ingestion.
Turns brown or black in contact with sulfur containing compounds.
Additional Information
° H. Kuhn, "Verdigris and Copper Resinate", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
Additional Images
Authority
- Artists' Pigments: A Handbook of their History and Characteristics, Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993 Comment: H. Kuhn, "Verdigris and Copper Resinate"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment'
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: Pigments Through the Ages - http://webexhibits.org/pigments/indiv/history/verdigris.html - alpha = 1.53; gamma = 1.56