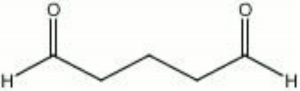
Glutaraldehyde
Description
A strong smelling gas sold commercially as a 25% aqueous solution. Glutaraldehyde polymerizes in water to form a clear viscous liquid. It is used as a biocide which acts by disrupting the cells of Lichen, mold, bacteria, and fungi by alkylating and crosslinking the proteins. Glutaraldehyde is also used as a synthetic tanning agent for leathers.
Synonyms and Related Terms
glutaric dialdehyde; 1,5-pentanedial; glutaral; 1,3-diformylpropane; Cidex; Glutarol; Novaruca; Verucasep; Ucaricide
Other Properties
Soluble in water.
| Composition | OHC(CH2)3CHO |
|---|---|
| CAS | 111-30-8 |
| Melting Point | -14 |
| Density | 0.7 |
| Molecular Weight | mol. wt. = 100.12 |
| Refractive Index | 1.4338 |
| Boiling Point | 187-189 (dec) |
Hazards and Safety
Toxic by inhalation and ingestion. Skin contact causes irritation.
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4480
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Glutaraldehyde (Accessed Mar. 1, 2006)