Difference between revisions of "Diethanolamine"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, viscous liquid surface active agent ([ | + | A colorless, viscous liquid surface active agent ([[surfactant]]) with a pungent odor. Diethanolamine is used in liquid [[nonionic detergent|nonionic detergents]], shampoos, cleaners, and polishes. Diethanolamine is also used as an [[emulsifier]], [[dispersant]], and [[plasticizer]] in some polymers. |
| − | |||
| − | |||
| + | See also [[monoethanolamine]], and [[triethanolamine]]. | ||
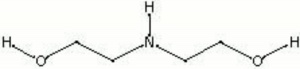
| + | [[[SliderGallery rightalign|diethanolamine.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
DEA; di(2-hydroxyethyl)amine; 2,2'-iminodiethanol; diethylolamine; bis(hydroxyethyl)amine | DEA; di(2-hydroxyethyl)amine; 2,2'-iminodiethanol; diethylolamine; bis(hydroxyethyl)amine | ||
| − | + | == Risks == | |
| − | == | + | * Combustible. Flash point = 134C. |
| + | * Skin irritant. | ||
| + | * Toxic by inhalation and ingestion. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=D45500&productDescription=DIETHANOLAMINE+PURIF+500ML&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Miscible in water, methanol, acetone. Slightly soluble in ether. | Miscible in water, methanol, acetone. Slightly soluble in ether. | ||
| Line 24: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 28.0 | + | | 28.0 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.088-1.092 | + | | 1.088-1.092 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 270 | + | | 270 C |
|} | |} | ||
| − | + | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 11:20, 21 July 2022
Description
A colorless, viscous liquid surface active agent (Surfactant) with a pungent odor. Diethanolamine is used in liquid nonionic detergents, shampoos, cleaners, and polishes. Diethanolamine is also used as an Emulsifier, Dispersant, and Plasticizer in some polymers.
See also Monoethanolamine, and Triethanolamine.
Synonyms and Related Terms
DEA; di(2-hydroxyethyl)amine; 2,2'-iminodiethanol; diethylolamine; bis(hydroxyethyl)amine
Risks
- Combustible. Flash point = 134C.
- Skin irritant.
- Toxic by inhalation and ingestion.
- ThermoFisher: SDS
Physical and Chemical Properties
Miscible in water, methanol, acetone. Slightly soluble in ether.
| Composition | NH(CH2CH2OH)2 |
|---|---|
| CAS | 111-42-2 |
| Melting Point | 28.0 C |
| Density | 1.088-1.092 g/ml |
| Molecular Weight | mol. wt. = 105.2 |
| Boiling Point | 270 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3156
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Teri Hensick, contributed information, 1998