Difference between revisions of "Sebacic acid"
Jump to navigation
Jump to search
(username removed) |
|||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
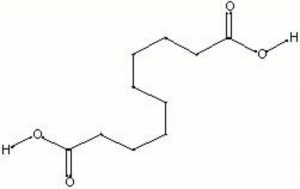
| − | + | [[[SliderGallery rightalign|sebacic acid.jpg~Chemical structure]]] | |
| − | White flaky crystals. Sebacic acid is obtained from heating [ | + | White flaky crystals. Sebacic acid is obtained from heating [[castor%20oil|castor oil]] with [[sodium%20hydroxide|sodium hydroxide]]. It is used in the synthesis of [[alkyd%20resin|alkyd]], [[nylon%20resin|nylon]], and [[polyester%20resin|polyester]] resins. Sebacic acid is also used as a [[plasticizer|plasticizer]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
1,8-octanedicarboxylic acid; sebacylic acid; decanedioic acid | 1,8-octanedicarboxylic acid; sebacylic acid; decanedioic acid | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Inhalation, ingestion and skin contact may cause irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96682.htm MSDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in ethanol, ether. Slightly soluble in water. | Soluble in ethanol, ether. Slightly soluble in water. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 133-134.5 | + | | 133-134.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.110-1.207 | + | | 1.110-1.207 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 295.0 | + | | 295.0 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8558 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8558 | ||
Latest revision as of 12:17, 16 June 2022
Description
White flaky crystals. Sebacic acid is obtained from heating Castor oil with Sodium hydroxide. It is used in the synthesis of alkyd, nylon, and polyester resins. Sebacic acid is also used as a Plasticizer.
Synonyms and Related Terms
1,8-octanedicarboxylic acid; sebacylic acid; decanedioic acid
Risks
- Combustible.
- Inhalation, ingestion and skin contact may cause irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in ethanol, ether. Slightly soluble in water.
| Composition | HOOC(CH2)8COOH |
|---|---|
| CAS | 111-20-6 |
| Melting Point | 133-134.5 C |
| Density | 1.110-1.207 g/ml |
| Molecular Weight | mol. wt.=102.07 |
| Refractive Index | 1.422 |
| Boiling Point | 295.0 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8558