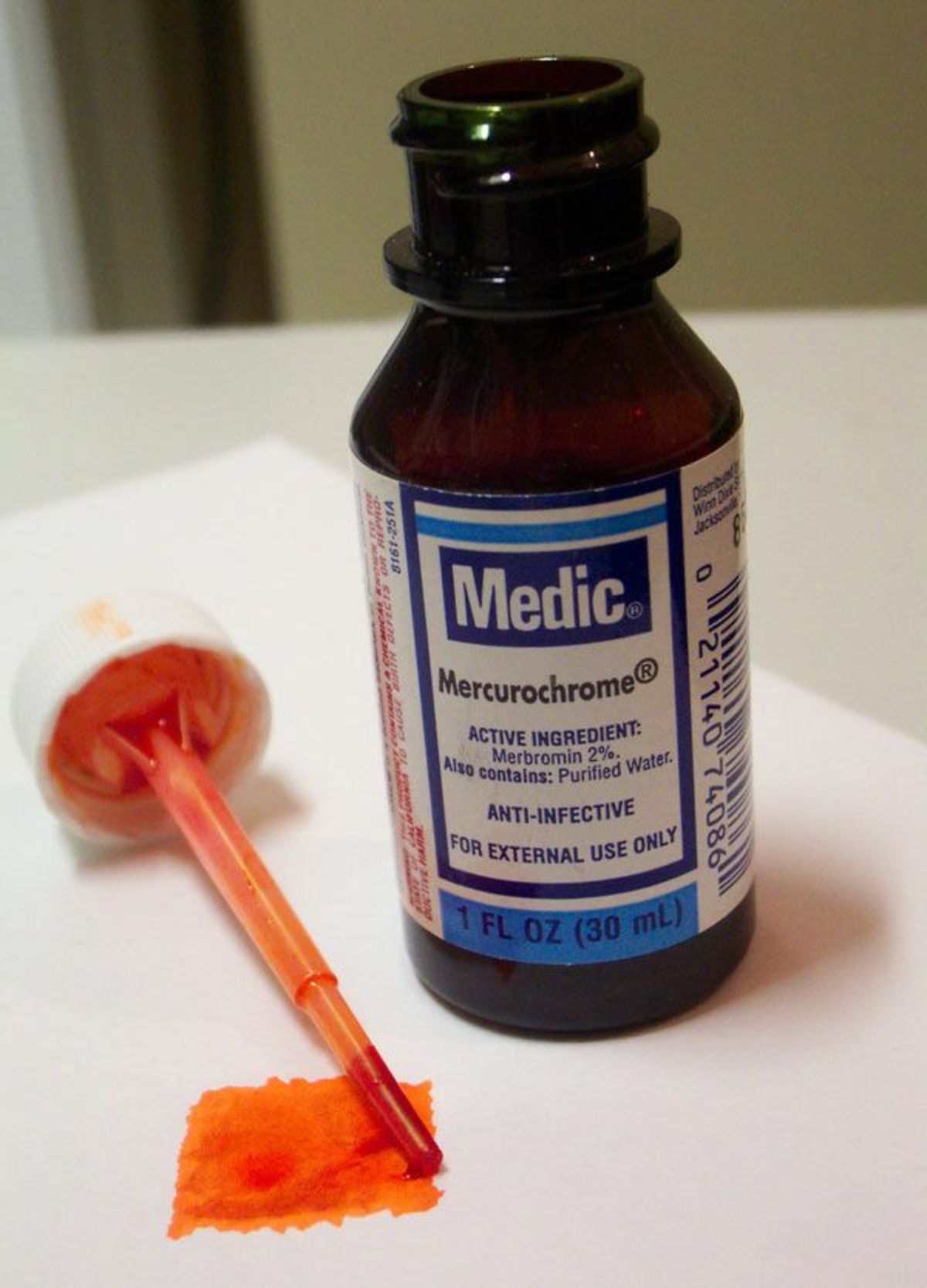
Merbromin
Description
An iridescent green crystalline compound composed of mercuric acetate dibromide fluorescein. Merbromin forms a red aqueous solution that fluoresces a bright yellow green. Since its discovery in 1919, merbromin was widely used as a germicide and antiseptic under the common brand name of Mercurochrome. In 1990, the U.S. banned its sale due to potential of mercury poisoning. Merbromin forms red stains that can be removed by washing first with permanganate solution and then with oxalic acid solution (Merck Index, 1996).
Synonyms and Related Terms
Mercurochrome; Merbromine; 'Monkey blood'; Asceptichrome; Cinfacromin; Supercrome orto; sodium mercurescein; mercuric acetate dibromide fluorescein; dibromohydroxymercurifluorescein disodium salt; 2,7-disodiumdibromo-4-hydroxymercurifluoescein
Risks
- Toxic by ingestion and inhalation.
- Contains mercury.
- Colors skin red.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water to form a carmine red solution. pH (0.5% solution) = 8.8
Insoluble in ethanol, acetone, chloroform, ether.
| Composition | C20H8Br2HgNa2O6 |
|---|---|
| CAS | 129-16-8 |
| Molecular Weight | mol. wt. = 750.7 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5914
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Wikipedia: http://en.wikipedia.org/wiki/Merbromin (Accessed Jan. 6 2006)