Difference between revisions of "Iron gall ink"
| (9 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:59.1008-SC25151.jpg|thumb|]] | + | [[File:59.1008-SC25151.jpg|thumb|Herd of Goats<br>MFA# 59.1008]] |
== Description == | == Description == | ||
| − | |||
| − | |||
| − | |||
[[File:ferrous sulfate mixture.jpg|thumb|Iron gall ink]] | [[File:ferrous sulfate mixture.jpg|thumb|Iron gall ink]] | ||
| + | The predominant black ink used in Europe from at least the Renaissance until it was replaced by synthetic inks in the late 19th century. Iron gall inks contain [[ferrous sulfate]] and [[gall]] extracts (gallotannic acid and [[gallic acid]]) in an aqueous solution of [[Gum arabic|gum Arabic]]. Gallotannic acid reacts with iron in solution to form iron gallotannate. The brown solution is used as an ink. As it dries on the paper or parchment, the ink oxidizes to a permanent deep blue-black color. [[Lampblack]], [[logwood]] (red), [[indigo]] (blue), or later, [[aniline black]], colorants were sometimes added to the ink to provide an initial dark color. Over time, iron gall inks can interact with paper causing severe degradation. [[Citric acid]], [[oxalic acid]], and [[potassium oxalate]] have been mentioned as sequestering agents to remove iron gall inks, but any addition of an acid to degraded paper or parchment is not recommended. Calcium phytate has be used as a [[chelating agent]]. | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | iron gallotannate; Eisengallustinte (Deut.); Gallustinte (Deut.); encre ferrogallique (Fr.); sidirogalliko melani (Gr.); inchiostro metallo gallico (It.); inchiostro di noce di galla (It.); ijzergallusinkt (Ned.); tinta ferrogálica (Port.); ferric tannate; iron tannate; acid ink; iron-gall ink; iron-gall nut ink | + | iron gallotannate; Eisengallustinte (Deut.); Gallustinte (Deut.); encre ferrogallique (Fr.); sidirogalliko melani (Gr.); inchiostro metallo gallico (It.); inchiostro di noce di galla (It.); ijzergallusinkt (Ned.); tinta ferrogálica (Port.); ferric tannate; iron tannate; acid ink; iron-gall ink; iron-gall nut ink; common ink; standard ink; oak gall ink; iron-based ink |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
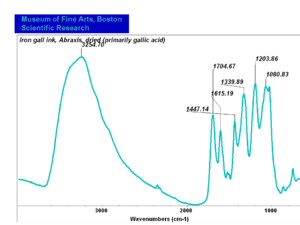
| − | == | + | [[[SliderGallery rightalign|Iron gall ink (primarily gallic acid).PNG~FTIR (MFA)]]] |
| + | == Physical and Chemical Properties == | ||
| − | * | + | * Iron-gallotannate is insoluble in water, ethanol, ether |
| + | * Some colored additives may be soluble in water and organic solvents. | ||
| + | * Some of the inks fade with time ranging from rusty brown to greenish to yellow. | ||
| + | * The presence of the acid can eat through paper | ||
| + | == Resources and Citations == | ||
| + | * Reissland, Birgit and Frank Ligterink (2011) [https://irongallink.org/ The Iron Gall Ink Website] Maintained by the Cultural Heritage Agency of the Netherlands. | ||
| + | * The Ink Corrosion Website at http://www.knaw.nl/ecpa/ink/intro.html | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 | ||
| − | |||
* ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | * ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Iron_gall_ink Iron_gall_ink] (Accessed Jan. 25, 2006 and March 2025) | |
| − | * Wikipedia | + | * AMOL reCollections Glossary - http://amol.org.au/recollections/7/i/htm |
| − | |||
| − | * | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | + | * Eric Hebborn, ‘The Art Forger’s Handbook’ The Overlook Press,Woodstock, NY 1997. | |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:33, 25 March 2025
Description
The predominant black ink used in Europe from at least the Renaissance until it was replaced by synthetic inks in the late 19th century. Iron gall inks contain Ferrous sulfate and Gall extracts (gallotannic acid and Gallic acid) in an aqueous solution of gum Arabic. Gallotannic acid reacts with iron in solution to form iron gallotannate. The brown solution is used as an ink. As it dries on the paper or parchment, the ink oxidizes to a permanent deep blue-black color. Lampblack, Logwood (red), Indigo (blue), or later, Aniline black, colorants were sometimes added to the ink to provide an initial dark color. Over time, iron gall inks can interact with paper causing severe degradation. Citric acid, Oxalic acid, and Potassium oxalate have been mentioned as sequestering agents to remove iron gall inks, but any addition of an acid to degraded paper or parchment is not recommended. Calcium phytate has be used as a Chelating agent.
Synonyms and Related Terms
iron gallotannate; Eisengallustinte (Deut.); Gallustinte (Deut.); encre ferrogallique (Fr.); sidirogalliko melani (Gr.); inchiostro metallo gallico (It.); inchiostro di noce di galla (It.); ijzergallusinkt (Ned.); tinta ferrogálica (Port.); ferric tannate; iron tannate; acid ink; iron-gall ink; iron-gall nut ink; common ink; standard ink; oak gall ink; iron-based ink
Physical and Chemical Properties
- Iron-gallotannate is insoluble in water, ethanol, ether
- Some colored additives may be soluble in water and organic solvents.
- Some of the inks fade with time ranging from rusty brown to greenish to yellow.
- The presence of the acid can eat through paper
Resources and Citations
- Reissland, Birgit and Frank Ligterink (2011) The Iron Gall Ink Website Maintained by the Cultural Heritage Agency of the Netherlands.
- The Ink Corrosion Website at http://www.knaw.nl/ecpa/ink/intro.html
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Wikipedia: Iron_gall_ink (Accessed Jan. 25, 2006 and March 2025)
- AMOL reCollections Glossary - http://amol.org.au/recollections/7/i/htm
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Eric Hebborn, ‘The Art Forger’s Handbook’ The Overlook Press,Woodstock, NY 1997.