Difference between revisions of "Zinc oxide"
| Line 11: | Line 11: | ||
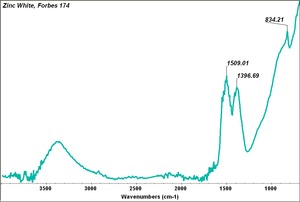
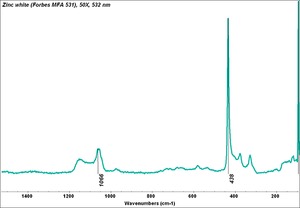
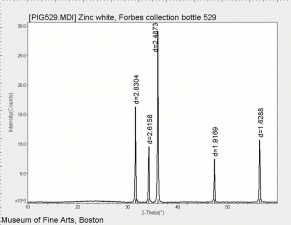
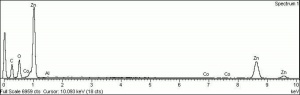
[[[SliderGallery rightalign|Zinc White, Forbes 174.TIF~FTIR (MFA)|Zinc white (Forbes MFA 531), 50X, 532 nm copy.tif~Raman (MFA)|PIG529.jpg~XRD (MFA)|f529sem.jpg~SEM (MFA)|f529edsbw.jpg~EDS(MFA)]]] | [[[SliderGallery rightalign|Zinc White, Forbes 174.TIF~FTIR (MFA)|Zinc white (Forbes MFA 531), 50X, 532 nm copy.tif~Raman (MFA)|PIG529.jpg~XRD (MFA)|f529sem.jpg~SEM (MFA)|f529edsbw.jpg~EDS(MFA)]]] | ||
| + | == Risks == | ||
| − | == | + | Noncombustible. Nonpoisonous, but slightly antiseptic. Inhalation or ingestion of dust may cause slight irritation. Zinc oxide fumes from firing may cause metal fume fever. Reacts violently with aluminum and magnesium powders. |
| + | |||
| + | Oil paints with zinc oxide may yellow and chalk with UV exposure. | ||
| + | |||
| + | U.S.Zinc: [http://www.uszinc.com/assets/uploads/2017/08/US-Zinc-SDS-ZnO-Rev10.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in acids and alkalis. Insoluble in water and ethanol. Normal zinc oxide contains rounded particles, precipitated acicular zinc oxide crystals are needle-like and crossed. | Soluble in acids and alkalis. Insoluble in water and ethanol. Normal zinc oxide contains rounded particles, precipitated acicular zinc oxide crystals are needle-like and crossed. | ||
| Line 40: | Line 46: | ||
| 2.00; 2.02 | | 2.00; 2.02 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
[[media:download_file_520.pdf|Characteristics of Common White Pigments]] | [[media:download_file_520.pdf|Characteristics of Common White Pigments]] | ||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 66: | Line 58: | ||
</gallery> | </gallery> | ||
| + | == Resources and Citations == | ||
| − | + | * H. Kuhn, "Zinc White", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | * | + | * Pigments Through the Ages. - http://webexhibits.org/pigments/indiv/technical/zincwhite.html |
| − | |||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Brass', 'Pigments' | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Brass', 'Pigments' | ||
| Line 85: | Line 76: | ||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 2.00; 2.02 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 2.00; 2.02 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Zinc_white (Accessed Nov. 29, 2005) |
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Revision as of 13:52, 27 October 2020
Description
A fine, white, insoluble powder. Zinc oxide is prepared by the oxidation of pure Zinc or by roasting zinc ore. It is used for a variety of purposes, however, the most important is as a paint pigment called Zinc white. The stable, opaque white powder is permanent and nontoxic. It was known since the Middle Ages but was rarely used as a pigment until 1834 when it was introduced as a watercolor pigment called Chinese white. By the turn of the century, zinc white had replaced Lead white in most paints, even though it had less covering power. Zinc oxide very strongly absorbs Ultraviolet radiation. Medicinally, zinc oxide is often used to treat rashes (e.g., Desinex); mixed with a small amount of iron oxide, it is sold as "Calamine" lotion. Zinc oxide is used as a pigment in oil paints, watercolor paints, ceramic glazes, printing inks, glass colorants, cosmetics, pharmaceuticals, ointments, and UV absorber.
Synonyms and Related Terms
zinc white; Chinese white; óxido de cinc (Esp.); oxyde de zinc (Fr.); blanc de zinc (Fr.); Zinkoxid (Deut.); Zinkweiss (Deut.); zinkoxide (Ned.); zinkwit (Ned.); ossido di zinco (bianco di zinco) (It.); leyko toy tsigkoy (Gr.); óxido de zinco (Port.); French zinc; snow white; philosophers' wool; nil alba; flowers of zinc; constant white, Hubbock's white; tutty
Risks
Noncombustible. Nonpoisonous, but slightly antiseptic. Inhalation or ingestion of dust may cause slight irritation. Zinc oxide fumes from firing may cause metal fume fever. Reacts violently with aluminum and magnesium powders.
Oil paints with zinc oxide may yellow and chalk with UV exposure.
U.S.Zinc: SDS
Physical and Chemical Properties
Soluble in acids and alkalis. Insoluble in water and ethanol. Normal zinc oxide contains rounded particles, precipitated acicular zinc oxide crystals are needle-like and crossed.
Birefringence is low. First order interference colors.
Fluoresces yellow
| Composition | ZnO |
|---|---|
| CAS | 1314-13-2 |
| Melting Point | 1975 |
| Density | 5.47-5.65 |
| Molecular Weight | mol. wt. = 81.4 |
| Refractive Index | 2.00; 2.02 |
Comparisons
Characteristics of Common White Pigments
Additional Images
Resources and Citations
- H. Kuhn, "Zinc White", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Pigments Through the Ages. - http://webexhibits.org/pigments/indiv/technical/zincwhite.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Brass', 'Pigments'
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 5.65 and ref.index = 2.00; 2.02
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 2.00; 2.02
- Wikipedia: http://en.wikipedia.org/wiki/Zinc_white (Accessed Nov. 29, 2005)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989