Difference between revisions of "Alizarin, synthetic"
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Uemura 07-23-2009 231.jpg|thumb|two sample of synthetic alizarin dyed silk<br>Uemera Dye Archive]] | ||
== Description == | == Description == | ||
A synthetic form of alizarin (1,2-dihydroxyanthraquinone) was first made in 1868 by Carl Graebe and Carl Lieberman, from [[anthracene|anthracene]], a coal tar product. Prior to that time, alizarin was obtained from the root of the madder plant, ''Rubia tinctorum'' L.. The orthorhombic, orange-red crystals of alizarin appear brownish-yellow in powder form. Alizarin is most commonly used for the derivatization of other dyes, but it is also known as a textile dye, a pigment, and an indicator. To prepare alizarin crimson pigment, alizarin is precipitated on a neutral base of [[alumina%20trihydrate|alumina trihydrate]] producing a brilliant, transparent color that has fair to good permanency. Alizarin will turn blue to purple in basic aqueous solutions, but it is more often used as an acid-base indicator in alcoholic solutions (0.5%) where it changes from yellow at pH 5.5 to red at pH 6.8. Alizarin is also used in spot tests as a reagent for [aluminum|aluminum]], [[indium|indium]], [[mercury|mercury]], [[zinc|zinc]], and [[zirconium|zirconium]]. As a textile dye, the cloth ([[cotton|cotton]], [[wool|wool]], or [[silk|silk]]) must be mordanted with a metal oxide. The shade produced depends on the metal present: aluminum yields a red; iron, a dark violet; and [[chromium|chromium]], a reddish-brown. | A synthetic form of alizarin (1,2-dihydroxyanthraquinone) was first made in 1868 by Carl Graebe and Carl Lieberman, from [[anthracene|anthracene]], a coal tar product. Prior to that time, alizarin was obtained from the root of the madder plant, ''Rubia tinctorum'' L.. The orthorhombic, orange-red crystals of alizarin appear brownish-yellow in powder form. Alizarin is most commonly used for the derivatization of other dyes, but it is also known as a textile dye, a pigment, and an indicator. To prepare alizarin crimson pigment, alizarin is precipitated on a neutral base of [[alumina%20trihydrate|alumina trihydrate]] producing a brilliant, transparent color that has fair to good permanency. Alizarin will turn blue to purple in basic aqueous solutions, but it is more often used as an acid-base indicator in alcoholic solutions (0.5%) where it changes from yellow at pH 5.5 to red at pH 6.8. Alizarin is also used in spot tests as a reagent for [aluminum|aluminum]], [[indium|indium]], [[mercury|mercury]], [[zinc|zinc]], and [[zirconium|zirconium]]. As a textile dye, the cloth ([[cotton|cotton]], [[wool|wool]], or [[silk|silk]]) must be mordanted with a metal oxide. The shade produced depends on the metal present: aluminum yields a red; iron, a dark violet; and [[chromium|chromium]], a reddish-brown. | ||
| + | |||
| + | * See also [https://cameo.mfa.org/wiki/Category:Uemura_dye_archive '''Uemera Dye Archive'''] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
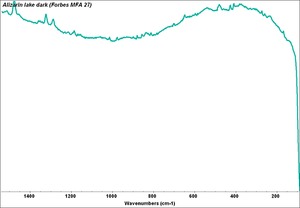
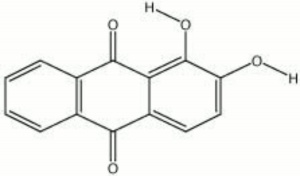
| + | [[[SliderGallery rightalign|Alizarin 2.TIF~FTIR (MFA)|Alizarin lake dark (Forbes MFA 27) resize.tif~Raman (MFA)|alizarin, synthetic.jpg~Chemical structure]]] | ||
1,2-dihydroxyanthraquinone; alizarine; Pigment Red 83 (calcium lake); Mordant Red 11; CI 58000; rouge d'alizarine (Fr.); Alizarin, synthetisch (Deut.); alizarina sintética (Esp.); alizarini, synthetiki (Gr.); alizarina sintetica (It.); alizarine (Ned.); alizarina, sintética (Port.); permanent violet; permanent crimson; alizarin crimson; crimson madder; | 1,2-dihydroxyanthraquinone; alizarine; Pigment Red 83 (calcium lake); Mordant Red 11; CI 58000; rouge d'alizarine (Fr.); Alizarin, synthetisch (Deut.); alizarina sintética (Esp.); alizarini, synthetiki (Gr.); alizarina sintetica (It.); alizarine (Ned.); alizarina, sintética (Port.); permanent violet; permanent crimson; alizarin crimson; crimson madder; | ||
| − | [ | + | == Risks == |
| + | |||
| + | Combustible. Slight potential for allergic reaction by contact, inhalation or ingestion. | ||
| + | |||
| + | ThermoFisher Scientific: [https://www.fishersci.com/store/msds?partNumber=AC400490250&productDescription=ALIZARIN+YELLOW+R%2C+96%25+%28+25GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Soluble in aromatic solvents, acetone, hot methanol and ether. | Soluble in aromatic solvents, acetone, hot methanol and ether. | ||
| Line 37: | Line 45: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''Artists' Pigments: A Handbook of their History and Characteristics'', Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: H.Schweppe, J.Winter, "Madder and Alizarin" | * ''Artists' Pigments: A Handbook of their History and Characteristics'', Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: H.Schweppe, J.Winter, "Madder and Alizarin" | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "alizarin" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "alizarin" [Accessed May 24, 2003]. Discovered 1868, first sold 1871 |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 65: | Line 67: | ||
* R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', ''Japanese Woodblock Prints'', Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: ISO rating | * R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', ''Japanese Woodblock Prints'', Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: ISO rating | ||
| − | * A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ''ICOM-CC Preprints'' Lyon, Getty Conservation Institute, Los Angeles | + | * A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ''ICOM-CC Preprints'' Lyon, Getty Conservation Institute, Los Angeles, 1999. p.654-660. |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * Website address | + | * Website address: www.straw.com/sig/dyehist |
| − | * | + | * Pigments Through the Ages: http://webexhibits.org/pigments/indiv/technical/alizarin.html ; RI=1.70 alizarin, 1.66 madder lake; |
* Colour Index International online at www.colour-index.org Comment: Structure; gives discoverer of calcium lake as Robequet & Colin 1826 | * Colour Index International online at www.colour-index.org Comment: Structure; gives discoverer of calcium lake as Robequet & Colin 1826 | ||
Latest revision as of 12:21, 22 June 2022
Description
A synthetic form of alizarin (1,2-dihydroxyanthraquinone) was first made in 1868 by Carl Graebe and Carl Lieberman, from Anthracene, a coal tar product. Prior to that time, alizarin was obtained from the root of the madder plant, Rubia tinctorum L.. The orthorhombic, orange-red crystals of alizarin appear brownish-yellow in powder form. Alizarin is most commonly used for the derivatization of other dyes, but it is also known as a textile dye, a pigment, and an indicator. To prepare alizarin crimson pigment, alizarin is precipitated on a neutral base of Alumina trihydrate producing a brilliant, transparent color that has fair to good permanency. Alizarin will turn blue to purple in basic aqueous solutions, but it is more often used as an acid-base indicator in alcoholic solutions (0.5%) where it changes from yellow at pH 5.5 to red at pH 6.8. Alizarin is also used in spot tests as a reagent for [aluminum|aluminum]], Indium, Mercury, Zinc, and Zirconium. As a textile dye, the cloth (Cotton, Wool, or Silk) must be mordanted with a metal oxide. The shade produced depends on the metal present: aluminum yields a red; iron, a dark violet; and Chromium, a reddish-brown.
- See also Uemera Dye Archive
Synonyms and Related Terms
1,2-dihydroxyanthraquinone; alizarine; Pigment Red 83 (calcium lake); Mordant Red 11; CI 58000; rouge d'alizarine (Fr.); Alizarin, synthetisch (Deut.); alizarina sintética (Esp.); alizarini, synthetiki (Gr.); alizarina sintetica (It.); alizarine (Ned.); alizarina, sintética (Port.); permanent violet; permanent crimson; alizarin crimson; crimson madder;
Risks
Combustible. Slight potential for allergic reaction by contact, inhalation or ingestion.
ThermoFisher Scientific: SDS
Physical and Chemical Properties
Soluble in aromatic solvents, acetone, hot methanol and ether.
Partially soluble in ethanol and water.
Alizarin does not fluoresce in ultraviolet light. Turns blue-purple in dilute alkaline solutions.
ISO R105 Lightfastness Classification = 4-5
| Composition | C14H6O2(OH)2 |
|---|---|
| CAS | 72-48-0 |
| Melting Point | 289-290 |
| Molecular Weight | mol. wt. = 240.22 |
| Boiling Point | 430 |
Resources and Citations
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: H.Schweppe, J.Winter, "Madder and Alizarin"
- Encyclopedia Britannica, http://www.britannica.com Comment: "alizarin" [Accessed May 24, 2003]. Discovered 1868, first sold 1871
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 475
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: Discovered 1868
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986 Comment: First appeared for sale in 1870
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: ISO rating
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999. p.654-660.
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address: www.straw.com/sig/dyehist
- Pigments Through the Ages: http://webexhibits.org/pigments/indiv/technical/alizarin.html ; RI=1.70 alizarin, 1.66 madder lake;
- Colour Index International online at www.colour-index.org Comment: Structure; gives discoverer of calcium lake as Robequet & Colin 1826